eGFR slope as a surrogate endpoint for clinical study in early stage of chronic kidney disease: from The Japan Chronic Kidney Disease Database
- PMID: 37466813
- PMCID: PMC10504220
- DOI: 10.1007/s10157-023-02376-4
eGFR slope as a surrogate endpoint for clinical study in early stage of chronic kidney disease: from The Japan Chronic Kidney Disease Database
Abstract
Background: In clinical trials targeting early chronic kidney disease (CKD), eGFR slope has been proposed as a surrogate endpoint for predicting end-stage kidney disease (ESKD). However, it is unclear whether the eGFR slope serves as a surrogate endpoint for predicting long-term prognosis in Japanese early CKD populations.
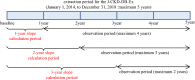
Methods: The data source was the J-CKD-Database, which contains real-world data on patients with CKD in Japan. eGFR slope was calculated from the eGFR of each period, 1-year (1-year slope), 2-year (2-year slope), and 3-year (3-year slope), for participants with a baseline eGFR ≥ 30 ml/min/1.73 m2. The outcome was ESKD (defined as dialysis initiation or incidence of CKD stage G5). The relationship between eGFR slope and the sub-distribution hazard ratio (SHR) of ESKD with death as a competing event was investigated using a Fine-Gray proportional hazard regression model.
Results: The number of participants and mean observation periods were 7768/877 ± 491 days for 1-year slope, 6778/706 ± 346 days for 2-year slope, and 5219/495 ± 215 days for 3-year slope. As the eGFR slope decreased, a tendency toward a lower risk of ESKD was observed. Compared with the 1-year slope, there was a smaller variation in the slope values for the 2-year or 3-year slope and a greater decrease in the SHR; therefore, a calculation period of 2 or 3 years for the eGFR slope was considered appropriate.
Conclusion: Even in Japanese patients with early stage CKD, a slower eGFR slope calculated from eGFR values over 2-3 years was associated with a decreased risk of ESKD.
Keywords: Chronic kidney disease; End stage kidney disease; Surrogate endpoint; eGFR slope.
© 2023. The Author(s).
Conflict of interest statement
The contributing authors reported the following financial supports: Seiji Itano, Eiichiro Kanda, and Hajime Nagasu have nothing to declare regarding potential conflicts of interest relevant to this article. Masaomi Nangaku has received lecture fees from Kyowa Kirin Co., Ltd., Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corporation, Bayer Yakuhin, Ltd., and Japan Tobacco Inc.; fee for writing manuscript from Kyowa Kirin Co., Ltd.; research funds from EPS Corporation, Parexel International Inc., and Japan Tobacco Inc.; and research grants from Kyowa Kirin Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Chugai Pharmaceutical Co., Ltd., Torii Pharmaceutical Co., Ltd., and Daiichi Sankyo Co., Ltd. Naoki Kashihara has received lecture fees from Daiichi Sankyo Co., Ltd., AstraZeneca K.K., Mitsubishi Tanabe Pharma Corporation, Ono Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd, Astellas Pharma Inc., Kyowa Kirin Co., Ltd., Bayer Yakuhin, Ltd., Nobelpharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Novartis Pharma K.K.; research funds from AstraZeneca K.K., Nobelpharma Co., Ltd., Daiichi Sankyo Co., Ltd., and Bayer Yakuhin, Ltd.; and research grants from Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Ono Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Astellas Pharma Inc., Otsuka Pharmaceutical Co., Ltd., and Boehringer Ingelheim GmbH.
Figures


Similar articles
-
eGFR slope as a surrogate endpoint for end-stage kidney disease in patients with diabetes and eGFR > 30 mL/min/1.73 m2 in the J-DREAMS cohort.Clin Exp Nephrol. 2024 Feb;28(2):144-152. doi: 10.1007/s10157-023-02408-z. Epub 2023 Oct 9. Clin Exp Nephrol. 2024. PMID: 37806976 Free PMC article.
-
Importance of glomerular filtration rate change as surrogate endpoint for the future incidence of end-stage renal disease in general Japanese population: community-based cohort study.Clin Exp Nephrol. 2018 Apr;22(2):318-327. doi: 10.1007/s10157-017-1463-0. Epub 2017 Sep 7. Clin Exp Nephrol. 2018. PMID: 28884361 Free PMC article.
-
Guidelines for clinical evaluation of chronic kidney disease in early stages : AMED research on regulatory science of pharmaceuticals and medical devices.Clin Exp Nephrol. 2024 Sep;28(9):847-865. doi: 10.1007/s10157-024-02514-6. Epub 2024 Jul 6. Clin Exp Nephrol. 2024. PMID: 38970650 Free PMC article.
-
Risk of Progression of Nonalbuminuric CKD to End-Stage Kidney Disease in People With Diabetes: The CRIC (Chronic Renal Insufficiency Cohort) Study.Am J Kidney Dis. 2018 Nov;72(5):653-661. doi: 10.1053/j.ajkd.2018.02.364. Epub 2018 May 18. Am J Kidney Dis. 2018. PMID: 29784612
-
Potential Role and Limitations of Estimated Glomerular Filtration Rate Slope Assessment in Cardiovascular Trials: A Review.JAMA Cardiol. 2022 May 1;7(5):549-555. doi: 10.1001/jamacardio.2021.5151. JAMA Cardiol. 2022. PMID: 34985495 Review.
Cited by
-
Predictive utility of nomogram based on serum glucose-regulated protein 78 and kidney function for long-term kidney graft survival.Sci Rep. 2024 Nov 21;14(1):28858. doi: 10.1038/s41598-024-80407-0. Sci Rep. 2024. PMID: 39572634 Free PMC article.
-
Comparative effects of sodium-glucose cotransporter 2 inhibitors versus dipeptidyl peptidase-4 inhibitors on kidney function decline in Japanese individuals with type 2 diabetes.Clin Exp Nephrol. 2024 Sep;28(9):894-901. doi: 10.1007/s10157-024-02499-2. Epub 2024 Apr 13. Clin Exp Nephrol. 2024. PMID: 38613740
-
Adding biomarker change information to the kidney failure risk equation improves predictive ability for dialysis dependency in eGFR <30 ml/min/1.73 m2.Clin Kidney J. 2024 Oct 24;17(11):sfae321. doi: 10.1093/ckj/sfae321. eCollection 2024 Nov. Clin Kidney J. 2024. PMID: 39564392 Free PMC article.
-
Chronic kidney disease risk assessment: Findings from backward-looking study using annual health check-up data in Japan.Diabetes Obes Metab. 2025 Jul;27(7):3686-3694. doi: 10.1111/dom.16390. Epub 2025 Apr 14. Diabetes Obes Metab. 2025. PMID: 40230182 Free PMC article.
-
Mathematical expansion and clinical application of chronic kidney disease stage as vector field.PLoS One. 2024 Mar 13;19(3):e0297389. doi: 10.1371/journal.pone.0297389. eCollection 2024. PLoS One. 2024. PMID: 38478478 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

