Early cessation of calcineurin inhibitors is feasible post-haploidentical blood stem cell transplant: the ANZHIT 1 study
- PMID: 37467011
- PMCID: PMC10514140
- DOI: 10.1182/bloodadvances.2023009840
Early cessation of calcineurin inhibitors is feasible post-haploidentical blood stem cell transplant: the ANZHIT 1 study
Abstract
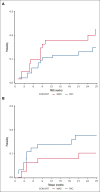
Haploidentical hematopoietic stem cell transplant (haplo-HSCT) using posttransplant cyclophosphamide (PTCy) is appropriate for those who lack matched donors. Most studies using PTCy have been retrospective making conclusions difficult. ANZHIT-1 was a phase 2 study conducted at 6 Australian allogeneic HSCT centers. The primary end points were disease-free and overall survival at 2 years after HSCT. The reduced-intensity conditioning (RIC) included fludarabine, cyclophosphamide, and 200 cGy total body irradiation, and the myeloablative conditioning (MAC) was IV fludarabine and busulfan. PTCy, MMF and a calcineurin inhibitor (CNI) were used for graft-versus-host disease (GVHD) prophylaxis. CNIs were weaned and ceased by day +120 in eligible patients on day 60. Patients (n = 78) with hematological malignancies were included in the study, with a median follow-up of 732 days (range, 28-1728). HSCT was RIC in 46 patients and MAC in 32 patients. Disease-free survival probability at 2 years was 67.5% (95% [CI], 53.2-85.6) for MAC recipients and 68.3% (95% CI, 56.3-83.01) for RIC recipients. Transplant-related mortality (TRM) on day 100 and year 1 was 4.9% (95% CI, 1.6-15.3) and 17.9% (95% CI, 8.8-36.5), respectively, in the MAC group compared with 3.1% (95% CI, 0.8.1-12) and 11.6% (95% CI, 6-22.4), respectively, in the RIC group. The median time for elective cessation of CNI was day 142.5 days, with no excess chronic GVHD (cGVHD) or mortality. Of the evaluable patients, 71.6% discontinued immunosuppression 12 months after transplant. This prospective haplo-HSCT trial using PTCY demonstrated encouraging survival rates, indicating that early CNI withdrawal is feasible and safe.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
-
- Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15(5):1767–1777. - PubMed

