Infections following bispecific antibodies in myeloma: a systematic review and meta-analysis
- PMID: 37467036
- PMCID: PMC10558589
- DOI: 10.1182/bloodadvances.2023010539
Infections following bispecific antibodies in myeloma: a systematic review and meta-analysis
Abstract
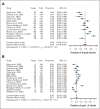
Bispecific antibodies, a novel immunotherapy with promising efficacy against multiple myeloma, form immune synapses between T-cell surface marker CD3 and malignant cell markers, including B-cell maturation antigen (BCMA), FcRH5, and G protein-coupled receptor GPRC5D. These bispecific antibodies so effectively deplete plasma cells (and to some extent T-cells) that patients are at increased risk of developing infections. A systematic review and meta-analysis of infections in published studies of patients with myeloma treated with bispecific antibodies was conducted to better characterize the infection risks. A literature search used MEDLINE, EMBASE, and Cochrane to identify relevant studies between inception and February 10, 2023, including major conference presentations. Phase 1b-3 clinical trials and observational studies were included. Sixteen clinical trials comprising 1666 patients were included. Median follow-up was 7.6 months and 38% of the cohort had penta-drug refractory disease. Pooled prevalence of all-grade infections was 56%, whereas the prevalence of grade ≥3 infections was 24%. Patients who were treated with BCMA-targeted bispecifics had significantly higher rates of grade ≥3 infections than non-BCMA bispecifics (25% vs 20%). Similarly, patients treated with bispecifics in combination with other agents had significantly higher rate of all-grade infection than those receiving monotherapy (71% vs 52%). In observational studies (n = 293), excluded from the primary analysis to ensure no overlap with patients in clinical trials, several infections classically associated with T-cell depletion were identified. This systematic review identifies BCMA-targeted bispecifics and bispecific combination therapy as having higher infection risk, requiring vigilant infection screening and prophylaxis strategies.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: E.R.S.C. receives research funding from Arnold Ventures (institutional). G.R. receives research funding from NHMRC. R.P. receives honoraria from Janssen, Celgene, GlaxoSmithKline, AbbVie, and Sanofi; holds a consulting or advisory role in GlaxoSmithKline, Celgene, Roche, BeiGene, and Janssen; and receives research funding from GlaxoSmithKline (institutional) and travel and accommodation expenses from Janssen and GlaxoSmithKline. B.W.T. discloses stock and other ownership interests in CSL Behring; research funding from MSD and Seqirus; and uncompensated relationships with CSL Behring (institutional), Takeda (institutional), and Moderna Therapeutics (institutional). A.S.K. receives research funding from Arnold Ventures (institutional). S.J.H. discloses having a leadership role at Haemalogix; receives honoraria from AbbVie, Amgen, Celgene/BMS, GKS, Janssen Cilag, Novartis, Roche, Genentech, Haemalogix, Eusa, and Terumo BCT; has a speaker’s bureau membership at AbbVie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, Roche Genetech, and Eusa; and research funding from Celgene/BMS, GSK, Janssen Cilag, and Haemalogix. The remaining authors declare no competing financial interests.
Figures

References
-
- Cliff ERS, Reynolds G, Popat R, Teh BW, Kesselheim AS, Mohyuddin GR. Acknowledging infection risk in bispecific antibody trials in the treatment of multiple myeloma. J Clin Oncol. 2023;41(10):1949–1951. - PubMed
-
- Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–45.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

