Rifaximin for prevention and treatment of hepatic encephalopathy in people with cirrhosis
- PMID: 37467180
- PMCID: PMC10360160
- DOI: 10.1002/14651858.CD011585.pub2
Rifaximin for prevention and treatment of hepatic encephalopathy in people with cirrhosis
Abstract
Background: Hepatic encephalopathy describes the spectrum of neuropsychiatric changes that may complicate the course of cirrhosis and detrimentally affect outcomes. Ammonia plays a key role in its development. Rifaximin is a non-absorbable antibiotic that inhibits urease-producing bacteria and reduces absorption of dietary and bacterial ammonia.
Objectives: To evaluate the beneficial and harmful effects of rifaximin versus placebo, no intervention, or non-absorbable disaccharides for: (i) the prevention of hepatic encephalopathy, and (ii) the treatment of minimal and overt hepatic encephalopathy, in people with cirrhosis, both when used alone and when combined with a non-absorbable disaccharide.
Search methods: We searched the Cochrane Hepato-Biliary Group Clinical Trials Register, CENTRAL, MEDLINE, Embase, three other databases, the reference lists of identified papers, and relevant conference proceedings. We wrote to authors and pharmaceutical companies for information on other published, unpublished, or ongoing trials. Searches were performed to January 2023.
Selection criteria: We included randomised clinical trials assessing prevention or treatment of hepatic encephalopathy with rifaximin alone, or with a non-absorbable disaccharide, versus placebo/no intervention, or a non-absorbable disaccharide alone.
Data collection and analysis: Six authors independently searched for studies, extracted data, and validated findings. We assessed the design, bias risk, and participant/intervention characteristics of the included studies. We assessed mortality, serious adverse events, health-related quality of life, hepatic encephalopathy, non-serious adverse events, blood ammonia, Number Connection Test-A, and length of hospital stay.
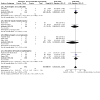
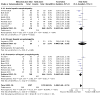
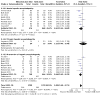
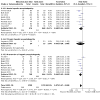
Main results: We included 41 trials involving 4545 people with, or at risk for, developing hepatic encephalopathy. We excluded 89 trials and identified 13 ongoing studies. Some trials involved participants with more than one type of hepatic encephalopathy or more than one treatment comparison. Hepatic encephalopathy was classed as acute (13 trials), chronic (7 trials), or minimal (8 trials), or else participants were considered at risk for its development (13 trials). The control groups received placebo (12 trials), no/standard treatment (1 trial), or a non-absorbable disaccharide (14 trials). Eighteen trials assessed rifaximin plus a non-absorbable disaccharide versus a non-absorbable disaccharide alone. We classified 11 trials as at high risk of overall bias for mortality and 28 for non-mortality outcomes, mainly due to lack of blinding, incomplete outcome data, and selective reporting. Compared to placebo/no intervention, rifaximin likely has no overall effect on mortality (risk ratio (RR) 0.83, 95% confidence interval (CI) 0.50 to 1.38; P = 48, I2 = 0%; 13 trials, 1007 participants; moderate-certainty evidence), and there may be no overall effect when compared to non-absorbable disaccharides (RR 0.99, 95% CI 0.49 to 1.97; P = 0.97, I2 = 0%; 10 trials, 786 participants; low-certainty evidence). However, there is likely a reduction in the overall risk of mortality when comparing rifaximin plus a non-absorbable disaccharide to a non-absorbable disaccharide alone (RR 0.69, 95% CI 0.55 to 0.86; number needed to treat for an additional beneficial outcome (NNTB) = 22; P = 0.001, I2 = 0%; 14 trials, 1946 participants; moderate-certainty evidence). There is likely no effect on the overall risk of serious adverse events when comparing rifaximin to placebo/no intervention (RR 1.05, 95% CI 0.83 to 1.32; P = 68, I2 = 0%; 9 trials, 801 participants; moderate-certainty evidence) and there may be no overall effect when compared to non-absorbable disaccharides (RR 0.97, 95% CI 0.66 to 1.40; P = 85, I2 = 0%; 8 trials, 681 participants; low-certainty evidence). However, there was very low-certainty evidence that use of rifaximin plus a non-absorbable disaccharide may be associated with a lower risk of serious adverse events than use of a non-absorbable disaccharide alone (RR 0.66, 95% CI 0.45 to 0.98; P = 0.04, I2 = 60%; 7 trials, 1076 participants). Rifaximin likely results in an overall effect on health-related quality of life when compared to placebo/no intervention (mean difference (MD) -1.43, 95% CI -2.87 to 0.02; P = 0.05, I2 = 81%; 4 trials, 214 participants; moderate-certainty evidence), and may benefit health-related quality of life in people with minimal hepatic encephalopathy (MD -2.07, 95% CI -2.79 to -1.35; P < 0.001, I2 = 0%; 3 trials, 176 participants). The overall effect on health-related quality of life when comparing rifaximin to non-absorbable disaccharides is very uncertain (MD -0.33, 95% CI -1.65 to 0.98; P = 0.62, I2 = 0%; 2 trials, 249 participants; very low-certainty evidence). None of the combined rifaximin/non-absorbable disaccharide trials reported on this outcome. There is likely an overall beneficial effect on hepatic encephalopathy when comparing rifaximin to placebo/no intervention (RR 0.56, 95% CI 0.42 to 0.77; NNTB = 5; P < 0.001, I2 = 68%; 13 trials, 1009 participants; moderate-certainty evidence). This effect may be more marked in people with minimal hepatic encephalopathy (RR 0.40, 95% CI 0.31 to 0.52; NNTB = 3; P < 0.001, I2 = 10%; 6 trials, 364 participants) and in prevention trials (RR 0.71, 95% CI 0.56 to 0.91; NNTB = 10; P = 0.007, I2 = 36%; 4 trials, 474 participants). There may be little overall effect on hepatic encephalopathy when comparing rifaximin to non-absorbable disaccharides (RR 0.85, 95% CI 0.69 to 1.05; P = 0.13, I2 = 0%; 13 trials, 921 participants; low-certainty evidence). However, there may be an overall beneficial effect on hepatic encephalopathy when comparing rifaximin plus a non-absorbable disaccharide to a non-absorbable disaccharide alone (RR 0.58, 95% CI 0.48 to 0.71; NNTB = 5; P < 0.001, I2 = 62%; 17 trials, 2332 participants; low-certainty evidence).
Authors' conclusions: Compared to placebo/no intervention, rifaximin likely improves health-related quality of life in people with minimal hepatic encephalopathy, and may improve hepatic encephalopathy, particularly in populations with minimal hepatic encephalopathy and when it is used for prevention. Rifaximin likely has no overall effect on mortality, serious adverse events, health-related quality of life, or hepatic encephalopathy compared to non-absorbable disaccharides. However, when used in combination with a non-absorbable disaccharide, it likely reduces overall mortality risk, the risk of serious adverse events, improves hepatic encephalopathy, reduces the length of hospital stay, and prevents the occurrence/recurrence of hepatic encephalopathy. The certainty of evidence for these outcomes is very low to moderate; further high-quality trials are needed.
Trial registration: ClinicalTrials.gov NCT00533910 NCT00298038 NCT02016196 NCT01769040 NCT04787276 NCT04736836 NCT02019784 NCT03077217 NCT02321371 NCT02190357 NCT01904409 NCT01842581 https://clinicaltrials.gov/ct2/show/NCT03515044 NCT02991612 NCT00686920 https://clinicaltrials.gov/ct2/show/NCT01676597 https://clinicaltrials.gov/ct2/show/NCT01846806 https://clinicaltrials.gov/show/nct01897051 https://clinicaltrials.gov/ct2/show/NCT01951209 https://clinicaltrials.gov/ct2/show/NCT02011841 https://clinicaltrials.gov/ct2/show/NCT02485106 https://clinicaltrials.gov/ct2/show/NCT03712280 NCT04159870 NCT03695705 NCT02488993 clinicaltrials.gov/ct2/show/NCT02508623 https://clinicaltrials.gov/ct2/show/NCT03069131 NCT04073290 NCT04775329 NCT05071716.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
HZ: has declared that they have no conflict of interest.
FK: has declared that they have no conflict of interest.
JT: has declared that they have no conflict of interest.
NK: reports being employed as a postdoctoral fellow in translational medicine by the University of Copenhagen, between 2016 and 2019 supported by the Novo Nordisk Foundation (grant no: NNF18SA0034956); she reports authorship of one of the trials included in this review (Kimer 2017) and of a previous systematic review and meta‐analysis of the effects of rifaximin in hepatic encephalopathy (Kimer 2014).
LG: reports receiving funding for research from Novo Nordisk, Alexion, Gilead, and Sobi International; she reports being a member of an advisory board for Novo Nordisk; she reports authorship of one of the trials included in this review (Kimer 2017) and of a previous systematic review and meta‐analysis of the effects of rifaximin in hepatic encephalopathy (Kimer 2014).
MM: has declared that they have no conflict of interest.
Figures




























































Update of
- doi: 10.1002/14651858.CD011585
Similar articles
-
L-ornithine L-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis.Cochrane Database Syst Rev. 2018 May 15;5(5):CD012410. doi: 10.1002/14651858.CD012410.pub2. Cochrane Database Syst Rev. 2018. PMID: 29762873 Free PMC article.
-
Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis.Cochrane Database Syst Rev. 2016 May 6;2016(5):CD003044. doi: 10.1002/14651858.CD003044.pub4. Cochrane Database Syst Rev. 2016. PMID: 27153247 Free PMC article.
-
Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis.Cochrane Database Syst Rev. 2016 Apr 18;4:CD003044. doi: 10.1002/14651858.CD003044.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2016 May 06;(5):CD003044. doi: 10.1002/14651858.CD003044.pub4. PMID: 27089005 Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Granulocyte colony-stimulating factor with or without stem or progenitor cell or growth factors infusion for people with compensated or decompensated advanced chronic liver disease.Cochrane Database Syst Rev. 2023 Jun 6;6(6):CD013532. doi: 10.1002/14651858.CD013532.pub2. Cochrane Database Syst Rev. 2023. PMID: 37278488 Free PMC article.
Cited by
-
Role of peripheral inflammation in minimal hepatic encephalopathy.Metab Brain Dis. 2024 Dec;39(8):1667-1677. doi: 10.1007/s11011-024-01417-5. Epub 2024 Aug 23. Metab Brain Dis. 2024. PMID: 39177864 Review.
-
Assessment of Access Barriers to Rifaximin Among Patients with Hepatic Encephalopathy Using Adjudicated Claims Data.Adv Ther. 2025 Jun;42(6):2739-2753. doi: 10.1007/s12325-025-03145-3. Epub 2025 Apr 7. Adv Ther. 2025. PMID: 40192964 Free PMC article.
-
Digestive dynamics: Unveiling interplay between the gut microbiota and the liver in macronutrient metabolism and hepatic metabolic health.Physiol Rep. 2024 Jun;12(12):e16114. doi: 10.14814/phy2.16114. Physiol Rep. 2024. PMID: 38886098 Free PMC article. Review.
-
Gut microbiome alterations and hepatic encephalopathy post-TIPS in liver cirrhosis patients.J Transl Med. 2025 Jul 4;23(1):745. doi: 10.1186/s12967-025-06774-y. J Transl Med. 2025. PMID: 40615853 Free PMC article.
-
Improved cognition after rifaximin treatment is associated with changes in intra- and inter-brain network functional connectivity.J Transl Med. 2024 Jan 12;22(1):49. doi: 10.1186/s12967-023-04844-7. J Transl Med. 2024. PMID: 38217008 Free PMC article.
References
References to studies included in this review
Ahmed 2018 {published data only (unpublished sought but not used)}
-
- Ahmed S, Ahmad S, Khan SU. Lactulose alone versus lactulose + rifaximin for the management of hepatic encephalopathy. Pakistan Journal of Medical and Health Sciences 2018;12(3):1269-71.
Ali 2014 {published data only (unpublished sought but not used)}
-
- Ali B, Zaidi YA, Alam A, Anjum HS. Efficacy of rifaximin in prevention of recurrence of hepatic encephalopathy in patients with cirrhosis of liver. Journal of the College of Physicians and Surgeons Pakistan 2014;24(4):269-73. [PMID: ] - PubMed
Babar 2017 {published data only (unpublished sought but not used)}
-
- Babar AN, Ali A, Khan MA, Tanveer A, ul Haq M. Rifaximin versus placebo preventing the recurrence of clinically overt hepatic encephalopathy in patients having cirrhosis of liver. Medical Forum 2017;28(12):15-8.
Bajaj 2011 {published data only}
-
- NCT00533910. Rifaximin in minimal hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT00533910 (First posted 24 September 2007).
Bajaj 2019 {published data only (unpublished sought but not used)}
-
- Bajaj J, Heimanson Z, Israel R. Rifaximin and lactulose combination therapy versus lactulose alone for prevention of overt hepatic encephalopathy recurrence: a pooled analysis of two randomized trials. Gastroenterology 2019;156(6):S560. [DOI: 10.1016/S0016-5085(19)38289-7] - DOI
-
- Bajaj J, Sanyal A, Sundaram V, Frenette C, Heimanson Z, Israel R, et al. Rifaximin plus lactulose is more efficacious than lactulose alone for risk reduction of overt hepatic encephalopathy (OHE) recurrence: a subgroup analysis by viral or alcohol cirrhosis etiology. American Journal of Gastroenterology 2021;116(Suppl 1):S570. [DOI: 10.14309/01.ajg.0000778480.00043.a7] - DOI
Bass 2004 {published and unpublished data}
-
- Bass NM, Ahmed A, Johnson L, Gardner JD. Rifaximin treatment is beneficial for mild hepatic encephalopathy. Hepatology 2004;40(4 Suppl):646A.
-
- Dimick-Santos L. Meeting of The Gastrointestinal Drugs Advisory Committee (GIDAC) to evaluate rifaximin 550 mg tablets for the proposed indication of maintenance of remission of hepatic encephalopathy (HE) in patients 18 years old and older. NDA 22-554 clinical review. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drug... (Accessed February 2014).
-
- Gardner J, Bass N, Johnson L, Ahmed A. The number connection test can predict the likelihood of asterixis and abnormal mental state in patients with hepatic encephalopathy treated with rifaximin. Hepatology 2004;40(4 Suppl 1):636-7A. [DOI: 10.1002/hep.1840400507] - DOI
-
- Salix Pharmaceuticals Inc. Xifaxan (rifaximin) tablets 550 mg for hepatic encephalopathy. Committee USFaDAGDA. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drug... AdvisoryCommittee/UCM203248.pdf ( Accessed February 2015).
-
- Xifaxan (rifaximin) tablets, 550 mg NDA 22-554. Briefing document for the gastrointestinal drugs advisory committee meeting 23 February 2010: FDA; 2010. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drug... ( Accessed February 2015).
Bass 2010 {published data only (unpublished sought but not used)}
-
- Alivisatos R. EC. 21-361 Xifaxan medical review part 6 access data FDA. Salix Pharmaceuticals, FDA. www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-361_Xifaxan_Medr_P6.pdf (Accessed February 2015).
-
- Bass N, Mullen K, Sigal S, Sanyal A, Poordad F, Merchant K, et al. Rifaximin is effective in maintaining remission in hepatic encephalopathy: results of a large, randomized, placebo-controlled trial. Journal of Hepatology 2009;50(Suppl 1):S39. [DOI: 10.1016/S0168-8278(09)60095-7] - DOI
-
- Brown RS, Bass N, Sanyal AJ, Poordad F, Sheikh MY, Mullen KD. Rifaximin significantly improved critical flicker frequency, and time-weighted CFF correlated with overt hepatic encephalopathy as assessed by Conn score in a 6 month study. Hepatology 2009;50(Suppl 4):449-50A. [DOI: 10.1002/hep.23301] - DOI
-
- Butterworth RF, Golden PL, Bortey E, Paterson C, Forbes WP. A critical flicker frequency value of 32 HZ predicts recurrence of overt hepatic encephalopathy in a double-blind, placebo controlled trial of rifaximin in patients with cirrhosis. Hepatology 2012;56(Suppl 1):916-7A.
Bucci 1993 {published and unpublished data}
Bureau 2021 {published data only (unpublished sought but not used)}
-
- Bureau C, Jezequel C, Archambault I, Thabut D, Dharancy S, Marc Perarnau J, et al. Rifaximin for the prevention of hepatic encephalopathy in patients treated by TIPS: a multicenter randomized placebo-controlled trial. Hepatology 2019;70(Suppl 1):10A.
-
- Bureau C, Thabut D, Jezequel C, Archambeaud I, D’Alteroche L, Dharancy S, et al. The use of rifaximin in the prevention of overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. A randomized controlled trial. Annals of Internal Medicine 2021;174(5):633-40. [DOI: 10.7326/M20-0202] [PMID: ] - DOI - PubMed
-
- NCT02016196. Double blind randomized study, comparing rifaximin vs placebo for the prevention of encephalopathy in patients treated by TIPS. clinicaltrials.gov/ct2/show/study/NCT02016196 (First posted 19 December 2013).
Butt 2018 {published data only (unpublished sought but not used)}
Fera 1993 {published and unpublished data}
-
- Fera G, Agostinacchio F, Nigro M, Schiraldi O, Ferrieri A. Rifaximin in the treatment of hepatic encephalopathy. European Journal of Clinical Research 1993;4(Suppl 1):57-66.
Festi 1993 {published and unpublished data}
-
- Festi D, Mazella G, Orsini M, Sottili S, Sangermano A, Bassi L, et al. Rifaximin in the treatment of chronic hepatic encephalopathy; results of a multicenter study of efficacy and safety. Current Therapeutic Research 1993;54(5):598-609.
Gill 2014 {published and unpublished data}
-
- Gill ML, Niaz T, Aziz H, Khan S. Outcomes of rifaximin plus lactulose versus lactulose alone in treatment of overt hepatic encephalopathy. Journal of Hepatology April 2014;60(Suppl 1):S215. [DOI: 10.1016/S0168-8278(14)60602-4] - DOI
Habib 2016 {published data only}
-
- Habib H, Nayab DE, Hayat Z, Jamil S, Khan H. Evaluation of efficacy of rifaximin in the treatment of hepatic encephalopathy in patients with cirrhosis. Gomal Journal of Medical Sciences 2016;14(1):37-40.
Hasan 2018 {published data only (unpublished sought but not used)}CTRI/2016/08/007215
-
- Hasan S, Datta S, Bhattacherjee S, Banik S, Saha S, Bandyopadhyay D. A randomized controlled trial comparing the efficacy of a combination of rifaximin and lactulose with lactulose only in the treatment of overt hepatic encephalopathy. Journal of the Association of Physicians of India 2018;66(1):32-6. [PMID: ] - PubMed
Higuera‐de‐la‐Tijera 2018 {published data only (unpublished sought but not used)}
-
- NCT02158182. Lactulose, L-ornithine L-aspartate, or rifaximin versus placebo for preventing hepatic encephalopathy in variceal bleeding. clinicaltrials.gov/show/NCT02158182 (First posted 6 June 2014).
-
- La Tijera FH, Servín-Caamaño AI, Abdo-Francis JM, Salas-Gordillo F, Pérez-Hernández JL, Camacho-Aguilera J, et al. A randomized, double-blind, clinical trial comparing lactulose, l-ornithine l-aspartate, or rifaximin, versus placebo, as primary prophylaxis of hepatic encephalopathy in cirrhotic patients with variceal bleeding. Journal of Hepatology 2017;66(Supplement 1):S49. [DOI: 10.1016/S0168-8278(17)30362-8] - DOI
-
- la Tijera FH, Servín-Caamaño AI, Salas-Gordillo F, Pérez-Hernández JL, Abdo-Francis JM, Camacho-Aguilera J, et al. Primary prophylaxis to prevent the development of hepatic encephalopathy in cirrhotic patients with acute variceal bleeding. Canadian Journal of Gastroenterology and Hepatology 2018;2018:1-10. [DOI: 10.1155/2018/3015891] [PMID: ] - DOI - PMC - PubMed
Kimer 2017 {published and unpublished data}EUCTR2012‐002890‐71‐DK
-
- EudraCT 2012-002890-71. Intestinal decontamination with rifaximin. Effects on the inflammatory and circulatory state in patients with cirrhosis and ascites - a randomised controlled clinical study. www.clinicaltrialsregister.eu/ctr-search/search?query=2012-002890-71 2012.
-
- Kimer N, Gluud LL, Pedersen JS, Tavenier J, Møller S, Bendtsen F. The psychometric hepatic encephalopathy syndrome score does not correlate with blood ammonia, endotoxins or markers of inflammation in patients with cirrhosis. Translational Gastroenterology and Hepatology 2021;6(8):1-9. [DOI: 10.21037/tgh.2020.02.14] [PMCID: PMC7724176] [PMID: ] - DOI - PMC - PubMed
-
- Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, et al. Rifaximin has limited effect on the inflammatory state and bacterial translocation in the patient with stable decompensated cirrhosis. Results from a randomized, placebo controlled trial. Hepatology 2016;64(Suppl 1):1041A. [DOI: 10.1002/hep.28799] - DOI
-
- Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, et al. Rifaximin has minor effects on bacterial composition, inflammation and bacterial translocation in cirrhosis; A randomized trial. Journal of Gastroenterology and Hepatology 2018;33(1):307-14. [DOI: 10.1111/jgh.13852] [PMID: ] - DOI - PubMed
Loguercio 2003 {published and unpublished data}
-
- Loguercio C, Federico A, De Girolamo V, Ferrieri A, Del Vecchio Blanco C. Cyclic treatment of chronic hepatic encephalopathy with rifaximin. Results of a double-blind clinical study. Minerva Dietologica e Gastroenterologica 2003;49:53-62. [PMID: ] - PubMed
Maharshi 2015 {published and unpublished data}
-
- Maharshi S, Sharma BC, Srivastava S, Jindal A. Prophylaxis of hepatic encephalopathy in acute variceal bleed in patients with cirrhosis: an open label randomized controlled trial of lactulose versus rifaximin. Journal of Clinical and Experimental Hepatology 2014;4(Suppl 2):S52-3.
-
- Maharshi S, Sharma BC, Srivastava S, Jindal A. Prophylaxis of hepatic encephalopathy in acute variceal bleed in patients with cirrhosis: An open label controlled trial of lactulose versus rifaximin. Journal of Hepatology 2014;60(Suppl):S59. [DOI: 10.1016/S0168-8278(14)60021-0] - DOI
Majeed 2018 {published data only (unpublished sought but not used)}
-
- Majeed A, Butt MW, Aziz HA. A comparison of rifaximin with placebo for the treatment of hepatic encephalopathy. Journal of Medical and Health Sciences 2018;12(1):362-4.
Manzhalii 2022 {published data only}
-
- NCT04787276. Efficacy and Safety of E.Coli Nissle 1917 in Patients With Mild (Stage 1-2) or Minimal Hepatic Encephalopathy. https://clinicaltrials.gov/ct2/show/NCT04787276 (First posted 8 March 2021).
Mas 2003 {published and unpublished data}
-
- Mas A, Rodés J, Sunyer L, Rodrigo L, Planas R, Vargas V, et al. Comparison of rifaximin and lactitol in the treatment of hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. Journal of Hepatology 2003;38:51-8. [DOI: 10.1016/s0168-8278(02)00350-1] [PMID: ] - DOI - PubMed
-
- Mas A. Treatment of hepatic encephalopathy (HE) with rifaximin compared with lactitol. Multicenter randomized, double-blind, double dummy study [Tratamiento de la encefalopatia hepatica (EH) con rifaximina en comparacion con lactitol. Estudio multicentrico aleatorizado, doble ciego, doble simulacion.]. Gastroenterologia y Hepatologia 1999;22(Suppl 1):40.
Massa 1993 {published and unpublished data}
-
- Massa P, Vallerino P, Dodero M. Treatment of hepatic encephalopathy with rifaximin. The European Journal of Clinical Research 1993;4:7-18.
Moneim 2021 {published data only}
-
- Moneim MA, Abdelaziz DH, Nagy YI, Baki AA, Attia AS, Sabry N. Rifaximin microbial resistance and its efficacy and safety as secondary prophylaxis of hepatic encephalopathy in patients with hepatitis C virus-related cirrhosis. International Journal of Clinical Practice 2021;75(11):1-11. [DOI: 10.1111/ijcp.14807] - DOI - PubMed
Muhammad 2016 {published data only}
-
- Muhammad N, Khan MAR, Hussain A. Role of rifaximin in preventing the recurrence of hepatic encephalopathy in patients with chronic liver disease. Pakistan Journal of Medical and Health Sciences 2016;10(4):1417-20.
Nawaz 2015 {published and unpublished data}
-
- Nawaz A, Ghafoor A, Khan A, Ullah H. Rifaximin versus placebo in reducing the risk of clinically overt hepatic encephalopathy recurrence in patients having cirrhosis of liver. Hepatology International 2015;9(Suppl 1):S104-5.
Paik 2005 {published and unpublished data}
-
- Kim MH, Han KH, Lee KS, Paik YH, Song KH, Na HK. Efficacy and safety of short-term administration of rifaximin in the treatment of hepatic encephalopathy. Korean Journal of Hepatology 2001;7(1):55-60. [onlinelibrary.wiley.com/o/cochrane/clcentral/articles/272/CN-01045272/fr...
-
- Song KH, Lee KS, Kim MH, Paik YH, Moon BS, Yoon SH, et al. The clinical efficacy of rifaximin in the treatment of hepatic encephalopathy (Comparison with lactulose). Hepatology 2000;32(4):407A.
Patel 2022 {published and unpublished data}2013‐004708‐20
-
- EUCTR2013-004708-20-GB. A trial to determine if rifaximin is more efficient than placebo in treating systemic inflammation and neutrophil (white blood cells) malfunction in patients with cirrhosis and chronic hepatic encephalopathy. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:201... (Registered 3 March 2014).
-
- NCT02019784. RIFSYS: randomised controlled trial of mechanistic effects of rifaximin in cirrhosis and chronic hepatic encephalopathy. clinicaltrials.gov/show/NCT02019784 (First posted 24 December 2013).
-
- Patel V, McPhail MJ, Zamalloa A, Støy S, Vijay GM, Huang X, et al. Results of a placebo-controlled double blind randomised trial to investigate the efficacy of rifaximin-alpha versus placebo in improving systemic inflammation in patients with cirrhosis and chronic hepatic encephalopathy (RIFSYS Trial). Journal of Hepatology 2018;68(Suppl 1):S107-8. [DOI: 10.1016/S0168-8278(18)30432-X] - DOI
-
- Patel V, Shawcross D, McPhail M. A placebo controlled single centre double blind randomised trial to investigate the efficacy of RIFaximin versus placebo in improving SYStemic inflammation and neutrophil malfunction in patients with cirrhosis and chronic hepatic encephalopathy. Scientific Report 2018:1-97.
Pawar 2019 {published data only (unpublished sought but not used)}
-
- Pawar V, Sonthalia N, Rathi P, Contractor Q, Surude R, Jain S, et al. Prospective randomized study of effect of lactulose or rifaximin or placebo in minimal hepatic encephalopathy. Indian Journal of Gastroenterology 2016;35(Suppl 1):A44. [DOI: 10.1007/s12664-016-0715-3] - DOI
-
- Pawar VB, Surude RG, Sonthalia N, Zanwar V, Jain S, Contractor Q, et al. Minimal hepatic encephalopathy in Indians: psychometric hepatic encephalopathy score and inhibitory control test for diagnosis and rifaximin or lactulose for its reversal. Journal of Clinical & Translational Hepatology 2019;7(4):304-12. [DOI: 10.14218/JCTH.2017.00037] [PMCID: PMC6943207] [PMID: ] - DOI - PMC - PubMed
Poudyal 2019 {published and unpublished data}
-
- Silwal NP, Kc S, Chaudhary S, Sharma M. Treatment outcome of hepatic encephalopathy in liver cirrhosis. Journal of Clinical and Experimental Hepatology 2018;8(S1):S51. [DOI: 10.1016/j.jceh.2018.06.363] - DOI
Riggio 2005 {published data only (unpublished sought but not used)}
-
- Masini A, Nicolao F, Efrati C, Merli M, Attili AF, Riggio O. Preliminary results of a randomised controlled trials in the prevention of early post-tips hepatic encephalopathy: a comparison between rifaximin and lactitol. Journal of Hepatology 2002;36(Suppl 1):205. [DOI: 10.1016/S0168-8278(02)80730-9] - DOI
-
- Riggio O, Masini A, Efrati C, Nicolao F, Angeloni S, Salvatori F, et al. Pharmacological prophylaxis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled study. Journal of Hepatology 2005;42(4):674-9. [DOI: 10.1016/j.jhep.2004.12.028] [PMID: ] - DOI - PubMed
Sharma 2013 {published data only (unpublished sought but not used)}CTRI/2013/07/003845
-
- NCT01218568. Rifaximin plus lactulose versus lactulose alone for the treatment of hepatic encephalopathy: a double blind randomized trial. clinicaltrials.gov/ct2/show/record/NCT01218568 (First posted 11 October 2010).
-
- Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomised double blind controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Journal of Clinical and Experimental Hepatology 2013;3(1S):S5-7. [DOI: 10.1016/j.jceh.2013.03.011] - DOI - PubMed
-
- Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. American Journal of Gastroenterology 2013;108(9):1458-63. [DOI: 10.1038/ajg.2013.219] [PMID: ] - DOI - PubMed
Sharma 2014 {published data only (unpublished sought but not used)}
-
- Sharma K, Pant S, Misra S, Dwiwedi M, Misra A, Narang S, et al. Effect of rifaximin, probiotics, and l-ornithine l-aspartate on minimal hepatic encephalopthy: a randomized controlled trial. Saudi Journal of Gastroenterology 2014;20(4):225-32. [DOI: 10.4103/1319-3767.136975] [PMCID: PMC4131305] [PMID: ] - DOI - PMC - PubMed
Sidhu 2011 {published data only}CTRI/2009/091/000979
-
- Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (The RIME trial). American Journal of Gastroenterology 2011;106:307-16. [DOI: 10.1038/ajg.2010.455] [PMID: ] - DOI - PubMed
Sidhu 2016 {published and unpublished data}CTRI/2010/091/003045
-
- Goyal O, Sidhu SS, Parker RA, Prasad J, Kishore H, Sood A, et al. Rifaximin versus lactulose in the treatment of minimal hepatic encephalopathy in patients with cirrhosis: a prospective, randomised, active-comparator, non-inferiority trial. Journal of Hepatology 2014;60(Suppl 1):S526.
-
- Goyal O, Sidhu S, Kishore H. Relapse of minimal hepatic encephalopathy after treatment in patients with liver cirrhosis. Hepatology International 2017;11(Suppl 1):S188-9. [DOI: 10.1007/s12072-016-9783-9] - DOI
-
- Wander P, Goyal O, Sidhu SS, Kishore H. Minimal hepatic encephalopathy in cirrhosis: how long to treat? Does minimal hepatic encephalopathy relapse after treatment? American Journal of Gastroenterology 2016;111(Suppl 1):S371.
Suzuki 2018 {published data only (unpublished sought but not used)}JapicCTI‐132086JapicCTI‐132087
-
- Phase II/III clinical trial of L-105 in patients with hepatic encephalopathy. www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?japicId=JapicCTI-132086 (First posted 19 March 2013).
-
- Phase III clinical trial of L-105 in patients with hepatic encephalopathy. www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?japicId=JapicCTI-132087 (First posted 19 March 2013).
-
- Suzuki K, Endo R, Takikawa Y, Moriyasu F, Aoyagi Y, Moriwaki H, et al. Efficacy and safety of rifaximin in Japanese patients with hepatic encephalopathy: a phase II/III, multicenter,randomized, evaluator-blinded, active-controlled trial and a phase III, multicenter, open trial. Hepatology Research 2018;48(6):411-23. [DOI: doi: 10.1111/hepr.13045] [PMID: ] - PubMed
Tan 2022 {published data only}
-
- NCT03077217. Low-dose Rifaximin in the Treatment of Covert Hepatic Encephalopathy. https://clinicaltrials.gov/ct2/show/NCT03077217 ( First posted 10 March 2017).
-
- Tan W, Wang J, Shi PM, Feng LM, Shi J, Ning BF, et al. Effects of low-dose and high-dose rifaximin in the treatment of covert hepatic encephalopathy. Journal of Clinical and Translational Hepatology 2022 ;10(6):1099-1106. [DOI: 10.14218/JCTH.2021.00457] [PMCID: PMC9634763] [PMID: ] - DOI - PMC - PubMed
Uthman 2020 {published data only}
-
- Uthman M, Mujtaba SWA, Qaisar AM. Examine the efficacy of rifaximin for hepatic encephalopathy patients with chronic liver disease. Medical Forum Monthly 2020;31(4):19-22.
Vyas 2017 {published data only (unpublished sought but not used)}
-
- NCT02321371. Effect of goal directed ammonia lowering therapy in acute on chronic liver failure patients with hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT02321371 (First posted 22 December 2014).
-
- Vyas T, Maiwall R, Jinadal A, Sarin S. Goal directed ammonia lowering therapy in acute on chronic liver failure (ACLF) with hepatic encephalopathy (HE): a randomized trial. Journal of Clinical and Experimental Hepatology 2017;7(Suppl 1):S1-21. [DOI: 10.1016/j.jceh.2017.01.026] [NCT: NCT02321371] - DOI
-
- Vyas TS, Jindal A, Maiwall R, Sahney A, Kalal C, Premkumar M, et al. Goal directed ammonia lowering therapy in acute on chronic liver failure (ACLF) with hepatic encephalopathy (HE): a randomized trial. Hepatology 2016;64(Suppl 1):704-5A. [DOI: 10.1002/hep.28799] - DOI
-
- Vyas TS, Sarin SK, Maiwall R. Effect of goal directed ammonia lowering therapy in acute-on-chronic liver failure patients with hepatic encephalopathy. Indian Journal of Gastroenterology 2016;35(Suppl):A67-8. [DOI: 10.1007/s12664-016-0715-3] - DOI
Wahib 2014 {published data only (unpublished sought but not used)}
Zeng 2021 {published data only (unpublished sought but not used)}
-
- NCT02190357. Rifaximin reduces the complications of decompensated cirrhosis: a randomized controlled trial. www.clinicaltrials.gov/ct2/show/NCT02190357 (First posted 15 July 2014).
References to studies excluded from this review
Abd‐Elsalam 2016 {published data only}
-
- Abd-Elsalam S, Ali LA, Soliman S, Ibrahim S, Elfert A. Randomized controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Journal of Hepatology 2016;64(S2):S211. [CLINICALTRIALS.GOV: NCT02120196] [DOI: 10.1016/S0168-8278(16)00176-8] - DOI - PubMed
-
- Elfert A, Abo Ali L, Soliman S, Ibrahim S, Abd-Elsalam S. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. European Journal of Gastroenterology & Hepatology 2016;28(12):1450-4. [DOI: 10.1097/MEG.0000000000000724] - DOI - PubMed
-
- NCT02120196. Comparative study of rifaximin versus norfloxacin in the secondary prophylaxis of spontaneous bacterial peritonitis (SBP). clinicaltrials.gov/ct2/show/NCT02120196 (First posted 22 April 2014).
Ahire 2017 {published data only}
-
- Ahire K, Sonawale A. Comparison of rifaximin plus lactulose with the lactulose alone for the treatment of hepatic encephalopathy. Journal of the Association of Physicians of India 2017;65:42-6. - PubMed
Ahluwalia 2014 {published data only}
-
- Ahluwalia V, Wade JB, Heuman DM, Hammeke TA, Sanyal AJ, Sterling RK, et al. Enhancement of functional connectivity, working memory and inhibitory control on multi-modal brain MR imaging with rifaximin in cirrhosis: implications for the gut-liver-brain axis. Metabolic Brain Disease 2014;29(4):1017-25. [DOI: 10.1007/s11011-014-9507-6] [PMCID: PMC4155029] [PMID: ] - DOI - PMC - PubMed
Anon 2014a {published data only}
-
- Hepatic encephalopathy. Rifaximin opens new perspectives. MMW Fortschritte der Medizin 2014;156(13):78-9. - PubMed
Anon 2014b {published data only}
-
- Intestine specific broad spectrum antibiotic. Rifaximin: therapy option in hepatic encephalopathy. MMW Fortschritte der Medizin 2014;156(3):64-5. - PubMed
Bajaj 2013 {published data only}
-
- Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLOS One 2013;8(4):e60042. [CLINICALTRIALS.GOV: NCT01069133] [DOI: 10.1371/journal.pone.0060042] - DOI - PMC - PubMed
Bajaj 2016b {published data only (unpublished sought but not used)}
-
- Bajaj J, Flamm SL, Chalassani NP, Diaz E, Kayali Z, Kang R, et al. Oral rifaximin soluble solid dispersion immediate release 40 mg prevents development of cirrhosis related complications: A phase 2, randomized, multicenter, double-blind, placebo-controlled trial. Hepatology 2016;64(Suppl 1):1027A.
-
- Kakiyama G, Pandak W, Heimanson Z, Zhou H, Thacker L, Zhao D, et al. Rifaximin solube solid dispersion tablets modify gut microbial function, as shown by increased faecal secondary bile acid levels compared with placebo, in patients with early decompensated cirrhosis. United European Gastroenterology Journal 2020;8(Suppl 8):611. [DOI: 10.1177/2050640620927345] - DOI
-
- Kakiyama G, Pandak W, Heimanson Z, Zhou H, Thacker L, Zhao D, et al. Rifaximin soluble solid dispersion tablets modify gut microbial function, as shown by increased fecal secondary bile acid levels compared with placebo, in patients with early decompensated cirrhosis. Gastroenterology 2020;158(6 Suppl 1):S1456. [DOI: 10.1016/S0016-5085(20)34307-9] - DOI
-
- NCT01904409. A randomized, double-blind, placebo-controlled, dose-ranging, multicenter study to assess the efficacy and safety of rifaximin soluble solid dispersion (SSD) tablets for the prevention of complications in subjects with early decompensated liver cirrhosis. clinicaltrials.gov/ct2/show/record/NCT01904409 (First 22 July 2013).
-
- Sanyal AJ, Chalassani N, Bajaj J, Diaz E, Kayali Z, Harmon M, et al. Impact of baseline chronic liver disease characteristics on the efficacy of oral rifaximin soluble solid dispersion tablets for the prevention of further decompensation or all-cause mortality in patients with cirrhosis and ascites. Hepatology 2016;64(Suppl 1):47A.
Bajaj 2020a {published data only}
-
- Bajaj J, Sundaram V, Frenette C, Heimanson Z, Israel R, Sanyal A. Lactulose withdrawal can potentiate breakthrough overt hepatic encephalopathy in patients controlled with rifaximin plus lactulose therapy: a post hoc analysis of a randomized controlled trial. Journal of Hepatology 2020;73(S1):S721. [CLINICALTRIALS.GOV: NCT01842581] [DOI: 10.1016/S0168-8278(20)31898-5] - DOI
-
- Flamm S, Mullen K, Heimanson Z, Zeev P, Sanyal A. Assessment of potential impact of demographic and baseline disease characteristics on rifaximin monotherapy versus lactulose combination therapy for the prevention of overt hepatic encephalopathy (OHE) recurrence. American Journal of Gastroenterology 2017;112(S1):S494. [DOI: 10.1038/ajg.2017.305] - DOI
-
- NCT01842581. The safety/efficacy of rifaximin with/without lactulose in participants with a history of recurrent hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT01842581 (First posted 29 April 2013).
-
- Sanyal A, Heimanson Z, Flamm S. SAT-110-Impact of rifaximin monotherapy or rifamixin plus lactulose on markers of inflammation in patients with cirrhosis and a history of hepatic encephalopathy. Journal of Hepatology 2019;70(Suppl 1):e677-8. [DOI: 10.1016/S0618-8278(19)31349-0] - DOI
-
- Sanyal A, Heimanson Z, Israel R, Mullen K, Flamm S. Prevention of overt hepatic encephalopathy recurrence with rifaximin alone versus rifaximin plus lactulose therapy: impact on quality of life and caregiver burden in patients with cirrhosis. Journal of Hospital Medicine 2018;13(4):1.
Bajaj 2020b {published data only}
-
- Bajaj JS, Rahimi RS, Hassanein T, Pyrsopoulos NT, Heimanson Z, Israel RJ, et al. Investigational water-soluble rifaximin formulation significantly shortens time to recovery in hospitalized patients with overt hepatic encephalopathy (OHE): a phase 2, randomized, double-blind, placebo-controlled trial. Hepatology 2020;72(Suppl 1):57-8A. [DOI: 10.1002/hep.31578] - DOI
-
- NCT03515044. Rifaximin Soluble Solid Dispersion (SSD) tablets plus lactulose for the treatment of overt hepatic encephalopathy (OHE). clinicaltrials.gov/ct2/show/study/NCT03515044 (First posted 2 May 2018).
Block 2010 {published data only}
-
- Block JP. Rifaximin improves the treatment of hepatic encephalopathy. Journal of Clinical Outcomes Management 2010;17(5):16-9.
Bohra 2020 {published data only}
Chang 2021 {published data only}
-
- Chang C, Huang CH, Tseng HJ, Yang FC, Chien RN. Real-world experience of the one-year efficacy of rifaximin add-on to lactulose is superior to lactulose alone in patients with cirrhosis complicated with recurrent hepatic encephalopathy in Taiwan. Journal of Personalized Medicine 2021;11(6):478. [PMID: 10.3390/jpm11060478] - DOI - PMC - PubMed
Cobbold 2018 {published and unpublished data}
-
- NCT01355575. Rifaximin in fatty liver disease (RiFL). clinicaltrials.gov/ct2/show/NCT01355575 First posted 18 May 2011.
Crisafulli 2016 {published data only}
-
- Crisafulli A, Demma S, Rigano G, Bertino G. Treatment with rifaximin high dose plus lactulose vs rifaximin standard dose plus lactulose for acute hepatic encephalopathy. Journal of Hepatology 2016;64(2):S258. [DOI: 10.1016/S0168-8278(16)00286-5] - DOI
CTRI/2019/05/018966 {published data only}
-
- CTRI/2019/05/018966. A double blind randomised controlled trial comparing lactulose plus rifaximin with lactulose plus rifaximin plus L-ornithin L-aspartate in the treatment of overt hepatic encephalopathy (grade III-grade IV) in patients with cirrhosis. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=32692&EncHid... (Registered 7 May 2019).
Danulescu 2013 {published data only}
-
- Danulescu RM, Ciobica A, Stanciu C, Trifan A. The role of rifaximine in the prevention of the spontaneous peritonitis. Revista Medico-Chirurgicala Societatii de Medici si Naturalisti din Iasi 2013;117(2):315-20. [PMID: ] - PubMed
De Marco 1984 {published data only}
-
- De Marco F, Santamaria Amato P, D'Arienzo A. Rifaximin in collateral treatment of portal systemic encephalopathy: a preliminary report. Current Therapeutic Research 1984;36(4):668-74.
Deshmukh 2016 {published data only (unpublished sought but not used)}
-
- Deshmukh PG, Konar A. An open label randomized study to evaluate the efficacy and safety of lactulose versus rifaximin in cirrhotics with covert hepatic encephalopathy. Indian Journal of Gastroenterology 2016;35(Suppl 1):LO-66.
Diana‐Maria 2019 {published data only}
-
- Diana-Maria S, Leahu A, Asaftei M, Garleanu I, Petrea O, Stanciu C, et al. Therapeutic approaches in patients with overt hepatic encephalopathy and liver cirrhosis. Journal of Gastrointestinal and Liver Diseases 2019;28(Suppl 1):68-9.
Di Piazza 1991 {published data only}
-
- Di Piazza S, Gabriella Filippazzo M, Valenze LM, Morello S. Rifaximin versus neomycine in the treatment of portosystemic encephalopathy. Italian Journal of Gastroenterology 1991;23(7):403-7. - PubMed
Dupont 2016 {published data only}
-
- DuPont HL, Flamm SL, Wolf RA, Sanyal AJ. Changes in antimicrobial susceptibility of fecal bacteria during rifaximin treatment for the prevention of overt hepatic encephalopathy (HE) recurrence. Hepatology 2016;64(Suppl 1):1047A.
-
- DuPont HL, Hassanein TI, Sanyal AJ, Wolf RA, Mullen KD. Genomic characterization of stool microbiota with rifaximin monotherapy versus lactulose in combination therapy for prevention of overt hepatic encephalopathy (HE) recurrence. Hepatology 2016;64(Suppl 1):720A.
EUCTR2014‐001856‐51‐DK {published data only}
-
- Anti-fibrotic and molecular aspects of rifaximin in alcoholic liver disease: A randomized placebo controlled clinical trial. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:201... (Date of registration 10 June 2014).
Frenette 2020a {published data only}
-
- Frenette C, Sundaram V, Heimanson Z, Israel R, Sanyal A. Lack of colonic microbial cross-resistance to other antibiotics in patients treated with rifaximin alone vs rifaximin plus lactulose for reducing the risk of overt hepatic encephalopathy (OHE) recurrence. American Journal of Gastroenterology 2020;115(Supplement):S517. [DOI: 10.14309/01.ajg.0000706100.58826.f6] - DOI
Frenette 2020b {published data only}
-
- Frenette C, Vinay S, Heimanson Z, Israel R, Sanyal A. Lactulose on intestinal microbial diversity and composition in patients with cirrhosis. American Journal of Gastroenterology 2020;115(Supplement):S517-8. [DOI: 10.14309/01.ajg.0000706104.30097.6e] - DOI
Gangarapu 2015 {published data only}
-
- Gangarapu V, Ince AT, Baysal B, Kayar Y, Kılıç U, Gök Ö, et al. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. European Journal of Gastroenterology and Hepatology 2015;27(7):840-5. [DOI: 10.1097/MEG.0000000000000348] [PMID: ] - DOI - PubMed
-
- NCT02009592. Efficacy of rifaximin on hepatosteatosis and steatohepatitis patients.. clinicaltrials.gov/ct2/show/NCT02009592 First posted 12 December 2013.
Giacomo 1993 {published data only}
-
- Giacomo F, Francesco A, Michele N, Oronzo S, Antonella F. Rifaximin in the treatment of hepatic encephalopathy. European Journal of Clinical Research 1993;4(Suppl 1):57-66.
Gupta 2021 {published data only}
-
- CTRI/2021/09/036321. Comparative study of Rifaximin alone versus combination therapy with Norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis with hepatic encephalopathy. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/09/036321 2021.
-
- Gupta T, Gaur V. Rifaximin alone versus combination with norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis with hepatic encephalopathy: a randomized-controlled trial. Hepatology (Baltimore, Md.) 2021;74(Suppl.1):1232A: 2073. [DOI: 10.1002/hep.32188] - DOI
Habib 2020 {published data only}
-
- Habib N, Amjad M, Afzal M, Iqbal MZ, Shehzad MA. Compare the efficacy of low versus high dose of rifaximin for primary prophylaxis of protosystemic encephalopathy. Pakistan Journal of Medical & Health Sciences 2020;14(3):843-5.
Hammond 2017 {published data only}
-
- Hammond DA, Dayama N, Martin BC. Impact of rifaximin and lactulose versus lactulose alone on hospitalization for acute recurrent hepatic encephalopathy. Value in Health 2017;20(5):A181.
Hotten 2003 {published data only}
-
- Hotten P, Marotta F, Naito Y, Minelli E, Helmy A, Lighthouse J, et al. Effects of probiotics, lactitol and rifaximin on intestinal flora and fecal excretion of organic acids in cirrhotic patients. Chinese Journal of Digestive Diseases 2003;4(1):13-8. [DOI: 10.1046/j.1443-9611.2003.00110.x] - DOI
Huang 2018 {published data only}
-
- Huang X, Chen S. Effect of rifaximin on gut microbiome in cirrhotic patients with variceal bleeding. Journal of Gastroenterology and Hepatology 2018;33(S4):53: OE-0898 (PD-0020). [DOI: 10.1111/jgh.14481] - DOI
-
- NCT02964195. Efficacy of rifaximin in treatment of cirrhotic gastroesophageal variceal hemorrhage: A multi-center randomized controlled clinical trial .. clinicaltrials.gov/ct2/show/NCT02964195 First posted 10 November 2016.
-
- NCT02991612. Efficacy of rafiximin in patients with cirrhotic gastroesophageal variceal hemorrhage: A single-center pilot study. clinicaltrials.gov/ct2/show/NCT02991612 First posted December 13, 2016: Sudy completed February 28, 2018.
Jain 2022 {published data only}
Jiménez 2022 {published data only}
-
- Jiménez C, Ventura-Cots M, Sala M, Calafat M, Garcia-Retortillo M, Cirera I, et al. Effect of rifaximin on infections, acute-on-chronic liver failure and mortality in alcoholic hepatitis: A pilot study (RIFA-AH). Liver International 2022;42(5):1109-20. [DOI: 10.1111/liv.15207] [PMID: ] - DOI - PMC - PubMed
John 2018 {published data only}
-
- John N, Aloysius M, Zaman M. Bovine immunoglobulin orally with modified doses of rifaximin for advanced cirrhotics in overt hepatic encephalopathy: a randomized placebo control prospective clinical pilot trial (BRAIN trial). Hepatology International 2018;12(2):S298. [DOI: 10.1007/s12072-018-9852-3] - DOI
Jones 2020 {published data only}
-
- Jones B, Berni E, Poole C, Jenkins-Jones S, Orr J, Amlani B, et al. Increased survival in patients with hepatic encephalopathy treated with rifaximin-alpha in combination with lactulose: an observational study from UK clinical practice. Journal of Hepatology 2020;73(Suppl 1):S37. [DOI: 10.1016/S0168-8278(20)30626-7] - DOI
Kaji 2017 {published data only}UMIN000029127
Kalambokis 2012a {published data only}
-
- Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, et al. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clinical Gastroenterology and Hepatology 2012;10(7):815-8. [DOI: 10.1016/j.cgh.2012.02.025] [PMID: ] - DOI - PubMed
Kalambokis 2012b {published data only}
-
- Kalambokis G, Tsianos EV. Thrompocytopenia associated with chronic liver disease: effects of rifaximin on platelet count. American Journal of Gastroenterology December 2010;105:2705-7. - PubMed
-
- Kalambokis, Mouzaki A, Rodi M, Tsianos EV. Rifaximin improves thrombocytopenia in patients with alcoholic cirrhosis in association with reduction of endotoxiemia. Liver International 2012;32(3):467-75. - PubMed
Kalambokis 2012c {published data only}
Kalambokis 2012d {published data only}
-
- Kalambokis GN, Tsianos EV. Rifaximin reduces endotoxemia and improves liver function and disease severity in patients with decompensated cirrhosis. Hepatology 2012;55(2):655-6. - PubMed
Kang 2017 {published data only}
-
- Kang SH, Lee YB, Lee JH, Nam JY, Chang Y, Cho H, et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Alimentary Pharmacology & Therapeutics 2017;46(9):845-5. [DOI: 10.1111/apt.14275] [PMID: ] - DOI - PubMed
Kawaratani 2022 {published data only}
-
- Kawaratani H, Kondo Y, Tatsumi R, Kawabe N, Tanabe N, Sakamaki A, et al. Long-term efficacy and safety of rifaximin in japanese patients with hepatic encephalopathy: a multicenter retrospective study. Journal of Clinical Medicine 2022;157110.3390/jcm11061571.(6):1571. [DOI: 10.3390/jcm11061571.] [PMCID: PMC8948903] [PMID: ] - DOI - PMC - PubMed
Khokhar 2015 {published data only}
-
- Khokhar N, Qureshi MO, Ahmad S, Ahmad A, Khan HH, Shafqat F, et al. Comparison of once a day rifaximin to twice a day dosage in the prevention of recurrence of hepatic encephalopathy in patients with chronic liver disease. Journal of Gastroenterology and Hepatology 2015;30:1420-2. [DOI: 10.111/jgh.12970] - DOI - PubMed
-
- Qureshi MO, Shafqat F, Salih, M, Khokhar N. Daily single dose rifaximin for prevention of hepatic encephalopathy in patients with chronic liver disease. Hepatology International 2015;9(Suppl 1):S325. - PubMed
Kimer 2018 {published data only}
-
- Kimer N, Gudmann NS, Pedersen J, Møller S, Nielsen MJ, Leeming DJ, et al. No effect of rifaximin on soluble CD163, mannose receptor or type III and IV neoepitope collagen markers in decompensated cirrhosis: Results from a randomized, placebo controlled trial. PLOS One 2018;13(9):1-12. [DOI: 10.1371/journal.pone.0203200] [EUDRACT: 2012-002890-71] - DOI - PMC - PubMed
Kimer 2022 {published data only}
-
- EUCTR2014-002264-33-DK. Rifaximin (an antibiotic) treatment of acute alcoholic liver injury. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2014-002264-33-DK 2014.
Kubota 2022 {published data only}
-
- UMIN000027511. Combination therapy with levocarnitine and rifaximin for hepatic encephalopathy in patients with cirrhosis: a randomized study. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031535 Registered 7 June 2017.
Lauridsen 2018 {published data only}
-
- Lauridsen MM, Gram J, Vilstrup H, Schaffalitzky de Muckadell OB. The continuous reaction times test for minimal hepatic encephalopathy validated by a randomized controlled multi-modal intervention. Journal of Clinical and Experimental Hepatology 2017;7(Suppl 1):S12-3. [DOI: 10.1016/j.jceh.2017.01.017] - DOI - PMC - PubMed
-
- Lauridsen MM, Gram J, Muckadell OB, Vilstrup H. The continuous reaction time test for minimal hepatic encephalopathy validated by a randomized controlled multimodal intervention. Journal of Hepatology 2018;68(Suppl 1):S740-1. [DOI: 10.1016/S0168-8278(18)31744-6] - DOI
-
- Lauridsen MM, Mikkelsen S, Svensson T, Holm J, Klüver C, Gram J, et al. The continuous reaction time test for minimal hepatic encephalopathy validated by a randomized controlled multi-modal intervention - a pilot study. PLOS One 2017;12(10):e0185412. [DOI: 10.1371/journal.pone.0185412] - DOI - PMC - PubMed
-
- NCT01773538. Diagnosis of covert/minimal hepatic encephalopathy by means of continuous reaction time measurement. clinicaltrials.gov/ct2/show/NCT01773538 (First posted 23 January 2013).
Lighthouse 2004 {published data only}
-
- Lighthouse J, Naito Y, Helmy A, Hotten P, Fuji H, Min CH, et al. Endotoxinemia and benzodiazepine-like substances in compensated cirrhotic patients: a randomized study comparing the effect of rifaximine alone and in association with a symbiotic preparation. Hepatology Research 2004;28(3):155-60. [DOI: 10.1016/j.hepres.2003.11.005] [PMID: ] - DOI - PubMed
Miglio 1997 {published data only}
Mohamed 2018 {published data only}
Mostafa 2015 {published data only}
Mullen 2014 {published data only}
-
- Mullen K, Sanyal A, Bass N, Poordad F, Huang S, Merchant K. The long term efficacy and safety of rifaximin in the maintenance of remission from overt hepatic encephalopathy in cirrhotic patients. Gastroenterology 2012;142(5 Suppl 1):S41-2. [DOI: 10.1016/S0016-5085(12)60157-7] - DOI
-
- Neff GW, Flamm SL, Mullen KD, Barrett AC, Bortey E. Improved outcomes in hepatic encephalopathy using rifaximin monotherapy compared to rifaximin and lactulose combination therapy. Gastroenterology 2013;144(Suppl 5):S-451. [DOI: 10.1016/S0016-5085(13)61665-0] - DOI
-
- Poordad F, Bass N, Sanyal AJ, Sheikh MY, Mullen KD, Sigal S, et al. The protective effect of rifaximin (1100 mg daily) from hepatic encephalopathy observed in a double-blind placebo controlled study is substantiated and durable over the long term. Hepatology 2009;50(Suppl 4):448-9A. [DOI: 10.1002/hep.23301] - DOI
-
- Sanyal A, Mullen KD, Bass NM, Frederick T, Poordad F, Hee J, et al. Rates of commonly occuring infections in cirrhosis patients remain stable during long-term rifaximin treatment. Journal of Hepatology 2012;56(Suppl 2):S255-6. [DOI: 10.1016/S0168-8278(12)60658-8] - DOI
NCT00364689 {published data only}
-
- RICE Trial: Rifaximin In Chronic Hepatic Encephalopathy - A Randomized, Controlled Trial. https://clinicaltrials.gov/show/NCT00364689.
NCT00748904 {published data only}
-
- NCT00748904. A Randomized Comparison of Rifaximin Versus Lactulose in Hospitalized Cirrhotic Patients With Progressive Renal Failure. ClinicalTrials.gov: https://www.clinicaltrials.gov/ct2/show/NCT00748904 First posted 9 September 2008.
NCT01676597 {published data only}
-
- NCT01676597. Efficacy of pentoxifylline and rifaximin combination in pentoxifylline refractory clinical and subclinical hepatopulmonary syndrome. clinicaltrials.gov/ct2/show/NCT01676597 (First posted 31 August 2012).
NCT01846806 {published data only}
-
- NCT01846806. The role of bacterial overgrowth and delayed intestinal transit in hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT01846806 (First posted 3 May 2013).
NCT01897051 {unpublished data only}
-
- NCT01897051. Rifaximin and propranolol combination therapy versus propranolol monotherapy in cirrhotic patients (RECOVER) [Hemodynamic response of rifaximin and non-selective β-blocker combination therapy versus non-selective β-blocker monotherapy in cirrhotic patients with esophageal varices]. clinicaltrials.gov/show/nct01897051 (First posted 11 July 2013).
NCT01951209 {published data only}
-
- NCT01951209. Pilot study of the effect of rifaximin on B-cell dysregulation in cirrhosis. clinicaltrials.gov/ct2/show/NCT01951209 (First posted 26 September 2013).
NCT02011841 {published data only}
-
- NCT02011841. Rifaximin for the secondary prevention of spontaneous bacterial peritonitis recurrence in cirrhotic patients. clinicaltrials.gov/ct2/show/NCT02011841 (First posted 13 December 2013).
NCT02485106 {published data only}
-
- NCT02485106. Rifaximin use in severe alcoholic hepatitis. clinicaltrials.gov/ct2/show/NCT02485106 (First posted 30 June 2015).
NCT03712280 {published data only}
-
- NCT03712280. MNK6106 for liver disease (hepatic cirrhosis) that in the past has affected the brain (hepatic encephalopathy). clinicaltrials.gov/ct2/show/NCT03712280 (First posted 19 October 2018).
NCT04159870 {published data only}
-
- NCT04159870. Rifaximin versus norfloxacin in the primary prophylaxis of spontaneous bacterial peritonitis. clinicaltrials.gov/ct2/show/NCT04159870 (First posted 12 November 2019).
Oey 2019 {published data only}
Orr 2016 {published data only}
Parini 1992 {published data only}
-
- Parini P, Cipolla A, Ronchi M, Salzetta A, Mazella G, Roda E. Effect of rifaximin and paromomycin in the treatment of portal-systemic encephalopathy. Current Therapeutic Research 1992;52(1):34-9.
Pedretti 1991 {published data only}
-
- Pedretti G, Calzetti C, Missale G, Fiaccadori F. Rifaximin versus neomycin on hyper-ammoniemia in chronic portal systemic encephalopathy of cirrhotics. A double-blind, randomized trial. Italian Journal of Gastroenterology 1991;23:175-8. - PubMed
Ponziani 2016 {published data only}
-
- Ponzani FR, Scaldaferri F, Pecere S, Sterbini F, Petito V, Lopetuso LR, et al. Increased abundance of beneficial bacteria is associated with clinical improvement after rifaximin treatment. Digestive and Liver Disease 2016;48(Suppl 2):E176-7. [DOI: 10.1016/S1590-8658(16)30264-X] - DOI
-
- Ponziani FR, Scaldaferri F, Sterbini FP, Petito V, Pecere S, Lopetuso LR, et al. Increased abundance of beneficial bacteria is associated with clinical improvement in patients receiving rifaximin treatment. United European Gastroenterology Journal 2016;4(Suppl 5):A649-50. [DOI: 10.1177/2050640616663689] - DOI
Pose 2020 {published data only}
-
- EUCTR2018-001698-25-GB. Efficacy of the combination of simvastatin plus rifaximin in patients with decompensated cirrhosis to prevent ACLF development: a multicenter, double-blind, placebo controlled randomized clinical trial. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:201... (Date of registration 01 October 2018).
-
- NCT03780673. Efficacy of the combination of simvastatin plus rifaximin in patients with decompensated cirrhosis to prevent ACLF development. clinicaltrials.gov/ct2/show/NCT03780673 (First posted 19 December 2018).
-
- Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. Gastroenterology and Hepatology 2020;5(1):31-41. [DOI: 10.1016/S2468-1253(19)30320-6] [PMID: ] - DOI - PubMed
-
- Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, et al. Safety and tolerability of two doses of simvastatin associated with rifaximin in patients with decompensated cirrhosis: a double‐blind, randomized, placebo‐controlled trial. Hepatology 2018;68(51):176A. [DOI: 10.1002/hep.30256] - DOI
Praharaj 2022 {published data only}
-
- NCT03695705. Rifaximin and norfloxacin for prevention of SBP in adults with decompensated cirrhosis. clinicaltrials.gov/ct2/show/NCT03695705 (First posted 4 October 2018).
-
- Praharaj D, Taneja S, Duseja A, Chawla YK, Dhiman RK. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis in cirrhotic patients. Indian Journal of Gastroenterology 2017;36(1):A57-8. [DOI: 10.1007/s12664-017-0798-5] - DOI
-
- Praharaj D, Taneja S, Duseja A, Chawla YK, Dhiman RK. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis (SBP) in cirrhotic patients. Journal of Clinical and Experimental Hepatology 2017;7(S2):S71. [DOI: 10.1016/j.jceh.2017.05.133] - DOI
-
- Praharaj D. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis (SBP) in cirrhotic patients. Hepatology International 2018;12(2):S553-4. [DOI: 10.1007/s12072-018-9852-3] - DOI
-
- Praharaj DL, Premkumar M, Roy A, Verma N, Taneja S, Duseja A, Dhiman RK. Rifaximin vs. norfloxacin for spontaneous bacterial peritonitis prophylaxis: A randomized controlled trial. Journal of Clinical and Experimental Hepatology 2022;12(2):336-342. [DOI: 10.1016/j.jceh.2021.08.010] [PMCID: PMC9077172] [PMID: ] - DOI - PMC - PubMed
Saboo 2021 {published data only}
Salehi 2019 {published data only}
-
- Salehi S, Lim S, Heneghan M, Aluvihare V, Heaton N, Patel V, et al. Rifaximin reduced the incidence of sepsis and all cause admissions whilst on the liver transplant waiting list. Journal of Hepatology 2018;68(Suppl 1):S119-20. [DOI: 10.1016/S0168-8278(18)30454-9] - DOI
-
- Salehi S, Tranah T, Lim S, Heaton N, Heneghan M, Aluvihare V, et al. Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list. Alimentary Pharmacology & Therapeutics 2019;50(4):435-41. [DOI: 10.1111/apt.15326] - DOI - PMC - PubMed
Sama 2004 {published data only}
-
- Sama C, Morselli-Labate AM, Pianta P, Lambertini L, Berardi S, Martini G. Clinical effects of rifaximin in patients with hepatic encephalopathy intolerant or nonresponsive to previous lactulose treatment: an open-label, pilot study. Current Therapeutic Research 2004;65(5):413-22. [DOI: 10.1016/j.curtheres.2004.10.002] - DOI - PMC - PubMed
Sarwar 2019 {published data only}
Schulz 2019 {published data only}
-
- Schulz C, Schütte K, Vilchez-Vargas R, Vasapolli R, Malfertheiner P. Long-term effect of rifaximin with and without lactulose on the active bacterial assemblages in the proximal small bowel and faeces in patients with minimal hepatic encephalopathy. Digestive Diseases 2019;37(2):161-9. [DOI: 10.1159/000494216] - DOI - PubMed
-
- Schulz C, Schütte K, Kropf S, Schmitt FC, Vasapolli R, Kliegis LM, et al. RiMINI - the influence of rifaximin on minimal hepatic encephalopathy (MHE) and on the intestinal microbiome in patients with liver cirrhosis: study protocol for a randomized controlled trial. Trials 2016;17(1):111. [DOI: 10.1186/s13063-016-1205-8] [EUDRA-CT: 2013-004414-18] [PMCID: PMC4770677] [PMID: ] - DOI - PMC - PubMed
Seifert 2021 {published data only}
-
- Seifert LL, Schindler P, Schoster M, Weller JF, Wilms C, Schmidt HH. Recurrence of hepatic encephalopathy after TIPS: effective prophylaxis with combination of lactulose and rifaximin. Journal of Clinical Medicine 2021;10(20):1-12. [DOI: 10.3390/jcm10204763] [PMCID: PMC8537523] [PMID: ] - DOI - PMC - PubMed
Song 2021 {published data only}
-
- Song DS, Yang JM, Jung YK, Yim H-J, Kim HY, Kim CW, et al. Rifaximin treatment in patients with severe alcoholic hepatitis; A multicenter, open-label, pilot randomized controlled trial. Hepatology (Baltimore, Md.) 2021;74(Suppl. 1):250A: 390. [DOI: 10.1002/hep.32188] - DOI
Suzuki 2019 {published data only}
Tatsumi 2021 {published data only}
Testa 1985 {published data only}
-
- Eftimiadi C, Deleo C, Schito GC. Treatment of hepatic encephalopathy with L/105, a new non-absorbable rifamycin. Drugs under Experimental and Clinical Research 1984;10(10):691-6. - PubMed
-
- Testa R, Eftimiadi C, Sukkar GS, De Leo C, Rovida S, Schito GC et al. A non-absorbable rifamycin for treatment of hepatic encephalopathy. Drugs Under Experimental and Clinical Research 1985;11(6):387-92. [PMID: ] - PubMed
Uchida 2020 {published data only}
UMIN000036998 {published data only}
-
- Randomized controlled trial comparing zinc-rifaximin combination therapy with zinc monotherapy in liver cirrhosis patients with minimal hepatic encephalopathy. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000042151 (Registered 10 June 2019). 2019. [UMIN: 000036998]
UMIN000038487 {published data only}UMIN000038487
-
- UMIN000038487. Use of rifaximin therapy in primary prevention of hepatorenal syndrome in cirrhotic patients with ascites. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000043845 (Registered 3 November 2019).
Venturini 2005 {published data only}
-
- Venturini I, Ferrieri A, Farina F, Avallone R, Corsi L, Baraldi M, et al. Evaluation of rifaximin, placebo and lactulose in reducing the levels of benzodiazepine-like compounds in patients with liver cirrhosis: A pilot study. Drugs Under Experimental and Clinical Research 2005;31(4):161-8. [PMID: ] - PubMed
Vittitow 2018 {published data only}
-
- Vittitow J, Heimanson Z, Israel R, Lowe E. Pharmacokinetics study of 2 investigational rifaximin soluble solid dispersion formulations (immediate release and sustained extended release) in healthy volunteers. Journal of Hepatology 2018;68(Suppl 1):S456. [DOI: 10.1016/S0168-8278(18)31175-9] - DOI
Vlachogiannakos 2013 {published data only}
Walker 2020 {published data only}
-
- Krag A, Schuchmann M, Sodatonou H, Pilot J, Whitehouse J, Strasser S, et al. Design of the Prospective Real-world Outcomes Study of hepatic encephalopathy Patients’ Experience on Rifaximin-α (PROSPER): an observational study among 550 patients. Hepatology, Medicine and Policy 2018;3(4):1-8. [DOI: 10.1186/s41124-017-0029-9] - DOI - PMC - PubMed
-
- Richard N, Pilot J, Holt A. PROSPER: Prospective Real World Outcomes Study of Hepatic Encephalopathy Patients' Experience on Rifaximin-a (TARGAXAN®/XIFAXAN®) 550 mg. clinicaltrials.gov/ct2/show/NCT02488993 (First posted 2 July 2015).
-
- Walker D. PROSPER study reveals reduction in bed days per hospitalisation and decreased risk of mortality for patients observed on rifaximin-alpha 550mg compared to a cohort on standard of care. Journal of Hepatology 2020;73(Suppl 1):S705. [DOI: 10.1016/S0168-8278(20)31873-0] - DOI
Williams 2000 {published data only}
-
- Williams R, James OFW, Warnes TW, Morgan MY. Evaluation of the efficacy of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. European Journal of Gastroenterology and Hepatology 2000;12(2):203-8. - PubMed
Yang 2016 {published data only}
-
- Yang J, Zhou SC, Zang JL, Yang YX. Clinical effects of ornithine aspartate combined with rifaximin in the treatment of acute hepatic encephalopathy. World Chinese Journal of Digestology 2016;24(3):415-9. [DOI: 10.11569/wcjd.v24.i3.415] - DOI
Zeng 2015 {published data only (unpublished sought but not used)}
-
- NCT02074280. Rifaximin predicts the complications of decompensated cirrhosis: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02074280 First posted 28 February 2014.
References to ongoing studies
ChiCTR1800018070 {published data only}
-
- ChiCTR1800018070. Clinical study of rehoci combined with long-acting octreotide in reducing the risk of rebleeding in patients with cirrhosis and portal hypertension. www.chictr.org.cn/showprojen.aspx?proj=30575 (Registered 29 August 2018).
EUCTR2014‐000102‐35‐IT {published data only}
-
- EUCTR2014-000102-35-IT. Effect of administration "add on" of Rifaximin on portal hypertension of patients with liver cirrhosis and esophageal varices in standard therapy with propranolol. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:201... (Date of registration 24 October 2014).
EUCTR2017‐000488‐34‐IT {published data only}EUCTR2017‐000488‐34‐IT
-
- EUCTR2017-000488-34-IT. Rifaximin blunted higher levels of endotoxin in cirrhosis patients: a randomized, double blind, short term interventional trial - rifaximin in cirrhotic patient. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:201... (Date of registration 8 June 2021).
EUDRACT2016‐002628‐96 {published data only}
-
- EUDRACT2016-002628-96. A multi-centre, double-blind, randomised, controlled clinical trial of Rifaximin to reduce infection in patients admitted to hospital with decompensated cirrhosis. www.clinicaltrialsregister.eu/ctr-search/search?query=2016-002628-96 (Date of registration September 2016).
-
- ISRCTN10994757. A multi-centre, double-blind, randomised, controlled clinical trial of Rifaximin to reduce infection in patients admitted to hospital with decompensated cirrhosis. www.isrctn.com/ISRCTN10994757 (Trial start date 15 April 2016).
NCT01846663 {published data only}
-
- NCT01846663. The efficacy, safety, and pharmacokinetics of rifaximin In subjects with severe hepatic impairment and hepatic encephalopathy. clinicaltrials.gov/ct2/show/NCT01846663 (First posted 3 May 2013).
NCT02439307 {published data only}
-
- NCT02439307. Effect of rifaximin on minimal hepatic encephalopathy and small intestinal bacterial overgrowth. clinicaltrials.gov/show/NCT02439307 (First posted 8 May 2015).
NCT02508623 {published data only}NCT02508623
-
- NCT02508623. Effect of administration of rifaximin on the portal pressure of patients with liver cirrhosis and esophageal varices (ERASE). clinicaltrials.gov/show/NCT02508623 (First posted 27 July 2015).
NCT02931123 (Riggio 2016) {published data only}
-
- NCT02931123. A RCT comparing lactulose and rifaximin associated with a vegetable diet in the preventions of post-TIPS overt hepatic encephalopathy. clinicaltrials.gov/show/NCT02931123 (First posted 12 October 2016).
NCT03069131 {published data only}
-
- NCT03069131. Two strategies of primary prophylaxis of spontaneous bacterial peritonitis in severe cirrhotic patients with ascites (ProPILARifax). clinicaltrials.gov/ct2/show/NCT03069131 (First posted 3 March 2017).
NCT04073290 (PEARL Study) {published data only}EUCTR2018‐004323‐37
-
- EUCTR2018-004323-37-NL. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt: a multi-centre randomized, double blind, placebo controlled trial. The PEARL trial. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:201... (Date of registration 07 January 2019). - PMC - PubMed
-
- NCT04073290. Prevention of post-TIPS hepatic encephalopathy by administration of rifaximin and lactulose (PEARL). clinicaltrials.gov/ct2/show/NCT04073290 (First posted 29 August 2019).
-
- Wit K, Schaapman JJ, Nevens F, Verbeek J, Coenen S, Cuperus FJ, et al. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial). BMJ Open Gastroenterology 2020;7(1):e000531. [DOI: 10.1136/bmjgast-2020-000531] [PMID: ] - DOI - PMC - PubMed
NCT04775329 {published data only}
-
- NCT04775329. Primary prophylaxis for spontaneous bacterial peritonitis (SIBOC). clinicaltrials.gov/ct2/show/NCT04775329 (First posted 1 March 2021).
NCT05071716 (RNLC3131) {published data only}
-
- NCT05071716. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of rifaximin soluble solid dispersion (SSD) for the delay of encephalopathy decompensation in cirrhosis. clinicaltrials.gov/ct2/show/NCT05071716 (First posted 8 October 2021).
TCTR20180509001 {published data only}
-
- TCTR20180509001. Comparison between rifaximin vs lactulose for treatment of hepatic encephalopathy. www.thaiclinicaltrials.org/show/TCTR20180509001 (Date of registration 09 May 2018).
Additional references
Acharya 2019
Amodio 2004
-
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metabolic Brain Disease 2004;19(3-4):253-67. [DOI: 10.1023/b:mebr.0000043975.01841.de] [PMID: ] - DOI - PubMed
Amodio 2015
Ampuero 2015
Arroyo 2020
Bai 2014
Bajaj 2007
-
- Bajaj JS, Saeian K, Verber MD, Hischke D, Hoffmann RG, Franco J, et al. Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. American Journal of Gastroenterology 2007;102(4):754-60. [DOI: 10.1111/j.1572-0241.2007.01048.x] - DOI - PubMed
Bajaj 2008
Bajaj 2009
Bajaj 2010a
Bajaj 2010b
Bajaj 2011a
-
- Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, et al. Review article: the design of clinical trials in hepatic encephalopathy - an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Alimentary Pharmacology & Therapeutics 2011;33(7):739-47. [PMID: ] - PMC - PubMed
Bajaj 2011b
-
- Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. American Journal of Gastroenterology 2011;106(9):1646-53. [DOI: 10.1038/ajg.2011.157] [PMCID: PMC3989143] [PMID: ] - DOI - PMC - PubMed
Bajaj 2012
-
- Bajaj JS, Gillevet PM, Patel NR, Ahluwalia V, Ridlon JM, Kettenmann B, et al. A longitudinal systems biology analysis of lactulose withdrawal in hepatic encephalopathy. Metabolic Brain Disease 2012;27:205-15. [PMID: ] - PubMed
Bajaj 2014
Bajaj 2016a
Bajaj 2021
-
- Bajaj JS, Shamsaddini A, Fagan A, Sterling R, Acharya C, Lee H, et al. Rifaximin and fecal microbiota transplant beneficially modulate gut metagenomic virulence factors in cirrhosis: analysis of two trials. Journal of Hepatology 2021;75(Suppl 2):S403-4. [DOI: 10.1016/S0168-8278(21)01843-2] - DOI
Berding 2009
-
- Berding G, Banati RB, Buchert R, Chierichetti F, Grover VP, Kato A, et al. Radiotracer imaging studies in hepatic encephalopathy: ISHEN practice guidelines. Liver International 2009;29:621-8. [PMID: ] - PubMed
Bircher 1966
Bircher 1982
-
- Bircher J, Bührer M, Franz K, Velthuijsen JA. 1st use of lactitol in the treatment of porto-systemic encephalopathy. Schweizerische Medizinische Wochenschrift 1982;112(38):1306-7. [PMID: ] - PubMed
Bjerring 2018
BNF 2021
-
- BMJ Group and the Royal Pharmaceutical Society of Great Britain. Rifaximin. In: British National Formulary. BMJ Group and the Royal Pharmaceutical Society of Great Britain, 2019. [ISBN: 978 0 85711 369 6]
Boutron 2021
-
- Boutron I, Page MJ, Higgins JP, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Brown 2006
Bustamante 1999
-
- Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. Journal of Hepatology 1999;30:890-5. [PMID: ] - PubMed
Calanni 2014
Caraceni 2021
Cheng 2021
Conn 1977
-
- Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology 1977;72:573-83. [PMID: ] - PubMed
Courson 2016
-
- Courson A, Jones GM, Twilla JD. Treatment of acute hepatic encephalopathy: comparing the effects of adding rifaximin to lactulose on patient outcomes. Journal of Pharmacy Practice 2016;29(3):212-7. - PubMed
Cudalbu 2019
Córdoba 2014
-
- Córdoba J, Ventura-Cots M, Simón-Talero M, Amorós À, Pavesi M, Vilstrup H, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). Journal of Hepatology 2014;60(2):275-81. [DOI: 10.1016/j.jhep.2013.10.004] [PMID: ] - DOI - PubMed
D'Amico 1986
-
- D'Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Digestive Diseases and Sciences 1986;31:468-75. - PubMed
D'Amico 2006
Dalal 2017
Das 2001
Deeks 2022
-
- Deeks J, Higgins J, Altman D. Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 6.3 edition. Cochrane, 2022.
Dhiman 2020
Di Pascoli 2017
DuPont 2016
-
- DuPont HL, Hassanein TI, Sanyal AJ, Wolf RA, Millen KD. Genomic characterization of stool microbiota with rifaximin monotherapy versus lactulose combination therapy for prevention of overt hepatic encephalopathy (HE) recurrence. Hepatology 2016;64(Suppl 1):720-1A. [DOI: 10.1002/hep.28799] - DOI
Dwan 2008
EASL and AASLD guideline 2014
-
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715-35. [DOI: 10.1002/hep.27210] [PMID: ] - DOI - PubMed
EASL Clinical Practice Guidelines 2022
Egger 1997
Elsaid 2020
Eltawil 2012
Fabrellas 2020
Facciorusso 2019
Faust 2020
Ferenci 2002
-
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002;35:716-21. [PMID: ] - PubMed
Fidel 2019
-
- Fidel KP, Payawal DA. The efficacy of rifaximin plus lactulose versus lactulose alone in the treatment of hepatic encephalopathy among patients with decompensated chronic liver disease: a meta-analysis. Hepatology International 2019;13(Suppl 1):S238. [DOI: 10.1007/s12072-019-09936-5] - DOI
Flamm 2018
-
- Flamm SL, Mullen KD, Heimanson Z, Sanyal AJ. Rifaximin has the potential to prevent complications of cirrhosis. Therapeutic Advances in Gastroenterology 2018;11:1756284818800307. [CLINICALTRIALS.GOV IDENTIFIER: NCT00298038] [DOI: 10.1177/1756284818800307] [PMCID: PMC6166307] [PMID: ] - DOI - PMC - PubMed
Fornio 2017
-
- Fonio P, Discalzi A, Calandri M, Doriguzzi Breatta A, Bergamasco L, Martini S, et al. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. La Radiologia medica 2017;122(9):713-21. [DOI: 10.1007/s11547-017-0770-6] - DOI - PubMed
Fu 2022
Gairing 2022
Gluud 2016
-
- Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD003044. [DOI: 10.1002/14651858.CD003044.pub3] - DOI - PMC - PubMed
Gluud 2017
-
- Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database of Systematic Reviews 2017, Issue 5(5):CD001939. Art. No: CD001939. [DOI: 10.1002/14651858.CD001939.pub4] [PMCID: 6481897] [PMID: ] - DOI - PMC - PubMed
Goel 2017
Goh 2018
GRADEpro [Computer program]
-
- GRADEpro. Brozek J, Oxman A, Schünemann H, Version 3.2 for Windows. Grade Working Group, 2008.
Groeneweg 1998
Grønkjær 2018
Guérit 2009
-
- Guérit JM, Amantini A, Fischer C, Kaplan PW, Mecarelli O, Schnitzler A, et al. Neurophysiological investigations of hepatic encephalopathy: ISHEN practice guidelines. Liver International 2009;29:789-96. [PMID: ] - PubMed
Han 2021
Higgins 2008
Higgins 2017
-
- Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2021a
-
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2021b
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hirode 2019
Huang 2007
Hudson 2019
ICH GCP 2016
-
- International Council for Harmonisation of technical requirements for pharmaceuticals for human use (ICH). ICH Harmonised Guideline. Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). database.ich.org/sites/default/files/E6_R2_Addendum.pdf (accessed 26 April 2022).
Iebba 2018
-
- Iebba V, Guerrieri F, Di Gregorio V, Levrero M, Gagliardi A, Santangelo F, et al. Combining amplicon sequencing and metabolomics in cirrhotic patients highlights distinctive microbiota features involved in bacterial translocation, systemic inflammation and hepatic encephalopathy. Scientific Reports 2018;8(1):8210. [DOI: 10.1038/s41598-018-26509-y] [PMCID: PMC5974022] [PMID: ] - DOI - PMC - PubMed
Jakobsen 2014
Jalan 2022
Jepsen 2010
-
- Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010;51:1675-82. - PubMed
Jeyaraj 2017
-
- Jeyaraj R, Morgan MY, Gluud LL. Aminoglycosides and metronidazole for people with cirrhosis and hepatic encephalopathy. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD012734. [DOI: 10.1002/14651858.CD012734] - DOI
Jiang 2008
-
- Jiang Q, Jiang XH, Zheng MH, Jiang LM, Chen YP, Wang L. Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. European Journal of Gastroenterology and Hepatology 2008;20:1064-70. - PubMed
Johanson 2007
Johnston 2021
-
- Johnston BC, Patrick DL, Devji T, Maxwell LJ, Bingham III CO, Beaton D, et al. Chapter 18: Patient-reported outcomes. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Kabeshova 2016
-
- Kabeshova A, Ben Hariz S, Tsakeu E, Benamouzig R, Launois R. Cost-effectiveness analysis of rifaximin-α administration for the reduction of episodes of overt hepatic encephalopathy in recurrence compared with standard treatment in France. Therapeutic Advances in Gastroenterology 2016;9(4):473-82. - PMC - PubMed
Kamal 2017
-
- Kamal F, Khan MA, Khan Z, Cholankeril G, Hammad T, Lee WM, et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: a systematic review and meta-analysis. European Journal of Gastroenterology & Hepatology 2017;29(10):1109-17. [DOI: 10.1097/MEG.0000000000000940] [PMID: ] - DOI - PubMed
Kimer 2014
Kircheis 2002
-
- Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Haussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology 2002;35:357-66. [PMID: ] - PubMed
Kircheis 2009
-
- Kircheis G, Knoche A, Hilger N, Manhart F, Schnitzler A, Schulze H, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology 2009;137:1706-15. [PMID: ] - PubMed
Komolafe 2020
-
- Komolafe O, Roberts D, Freeman S, Wilson P, Sutton A, Cooper N, et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD013125. [DOI: 10.1002/14651858.CD013125.pub2] [PMID: ] - DOI - PMC - PubMed
Lauridsen 2011
-
- Lauridsen MM, Jepsen P, Vilstrup H. Critical flicker frequency and continuous reaction times for the diagnosis of minimal hepatic encephalopathy: a comparative study of 154 patients with liver disease. Metabolic Brain Disease 2011;26:135-9. [PMID: ] - PubMed
Lawton 1939
-
- Lawton G. The measurement of adult intelligence. Baltimore: the Williams and Wilkins Company, 1939.
Leevy 2007
Levitt 2019
Mendoza 2020
Menshawy 2019
Miller 2014
Montagnese 2004
Montagnese 2014
Montagnese 2019
Moon 2023
-
- Moon AM, Kim HP, Jiang Y, Lupu G, Bissram JS, Barritt AS, Tapper EB. Systematic review and meta-analysis on the effects of lactulose and rifaximin on patient-reported outcomes in hepatic encephalopathy. American Journal of Gastroenterology 2023;118(2):284-293. [DOI: 10.14309/ajg.0000000000002008] [PMCID: PMC9904367] [PMID: ] - DOI - PMC - PubMed
Morgan 2016
Morgan 2018
-
- Morgan MY. Hepatic Encephalopathy in Patients with Cirrhosis . In: Dooley JS, Lok ASF, Garcia-Tsao G, Pinzani M, editors(s). Sherlock's Diseases of the Liver and Biliary System. 13 edition. Chichester, West Sussex: John Wiley & Sons Ltd, 2018:Chapter 10. [DOI: ]
Neff 2010
-
- Neff G. Pharmacoeconomics of hepatic encephalopathy. Pharmacotherapy 2010;30:28S-32S. [PMID: ] - PubMed
Neff 2013
Neff 2018
Nolte 1998
-
- Nolte W, Wiltfang J, Schindler C, Münke H, Unterberg K, Zumhasch U, et al. Portosystemic hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with cirrhosis: clinical, laboratory, psychometric, and electroencephalographic investigations. Hepatology 1998;28(5):1215-25. [DOI: 10.1002/hep.510280508] [PMID: ] - DOI - PubMed
O'Carroll 1991
-
- O'Carroll RE, Hayes PC, Ebmeier KP, Dougall N, Murray C, Best JJ, et al. Regional cerebral blood flow and cognitive function in patients with chronic liver disease. Lancet 1991;337:1250-3. [PMID: ] - PubMed
Orr 2014
Ortiz 2007
-
- Ortiz M, Córdoba J, Doval E, Jacas C, Pujadas F, Esteban R, et al. Development of a clinical hepatic encephalopathystaging scale. Alimentary Pharmacology& Therapeutics 2007;26(6):859-67. [DOI: ] - PubMed
Page 2014
-
- Page MJ, McKenzie JE, Kirkham J, Dwan K, Kramer S, Green S, et al. Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: MR000035. [DOI: 10.1002/14651858.MR000035.pub2] - DOI - PMC - PubMed
Page 2021
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Page 2021a
Page 2021b
Pantham 2017
Parsons‐Smith 1957
-
- Parsons-Smith BG, Summerskill WH, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet 1957;273:867-71. [PMID: ] - PubMed
Patidar 2015
Peryer 2021
-
- Peryer G, Golder S, Junqueira D, Vohra S, Loke YK. Chapter 19: Adverse effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Pimentel 2021
Poordad 2007
-
- Poordad FF. Review article: the burden of hepatic encephalopathy. Alimentary Pharmacology & Therapeutics 2007;25:3-9. [PMID: ] - PubMed
Qin 2014
Razzack 2021
-
- Razzack AA, Hussain N, Hassan SA, Gulzar R, Karam A, Panday P, et al. Rifaximin for prophylaxis of post-TIPPS hepatic encephalopathy-a meta analysis. Journal of Hepatology 2021;75(Suppl 2):S383. [DOI: 10.1016/S0168-8278(21)01843-2] - DOI
Reitan 1955
-
- Reitan RM. The relation of the trail making test to organic brain damage. Journal of Consulting Psychology 1955;19(5):393-4. [DOI: ] - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager (RevMan). Version 5.4. The Cochrane Collaboration, 2014.
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 1.22.0. Cochrane Collaboration, 2020.
Riggio 1990
-
- Riggio O, Varriale M, Testore GP, Di Rosa R, Di Rosa E, Merli M, et al. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. Journal of Clinical Gastroenterology 1990;12:433-6. [PMID: ] - PubMed
Riggio 2008
-
- Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A, et al. Incidence, natural history, and risk factors of hepatic encephalopathy aftertransjugular intrahepatic portosystemic shunt with polytetrafluoroethylenecoveredstent grafts. American Journal of Gastroenterology 2008;103(11):2738-46. [DOI: 10.1111/j.1572-0241.2008.02102.x] [PMID: ] - DOI - PubMed
Roman 2011
-
- Roman E, Córdoba J, Torrens M, Torras X, Villanueva C, Vargas V, et al. Minimal hepatic encephalopathy is associated with falls. American Journal of Gastroenterology 2011;106:476-82. - PubMed
Romero‐Gómez 2001
Rose 2020
Saunders 1981
Savovic 2012
-
- Savovic J, Jones H, Altman D, Harris RJ, Juni P, Pildal J et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technology Assessment 2012;16(35):1-82. - PubMed
Savovic 2018
-
- Savovic J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JPT, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane Reviews: The ROBES meta-epidemiologic study. American Journal of Epidemiology 2018;187(5):1113-22. [DOI: 10.1093/aje/kwx344] [PMID: ] - DOI - PMC - PubMed
Scarpignato 2005
Schomerus 1998
Schultz 2019
-
- Schulz C, Schütte K, Vilchez-Vargas R, Vasapolli R, Malfertheiner P. Long-term effect of rifaximin with and without lactulose on the active bacterial assemblages in the proximal small bowel and faeces in patients with minimal hepatic encephalopathy.. Digestive Diseases 2019;37(2):161-9. [DOI: 10.1159/000494216] [PMID: ] - DOI - PubMed
Schünemann 2021
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Shaheen 2019
Sharma 2007
Sharma 2009
Sharma 2010
-
- Sharma P, Sharma BC, Sarin SK. Prevalence of abnormal psychometric tests and critical flicker frequency after clinical recovery of overt hepatic encephalopathy. Neurology 2010;58:220-4. [PMID: ] - PubMed
Shrestha 2020
-
- Shrestha D, Rathi S, Grover S, Taneja S, Duseja A, Chawla YK, et al. Factors affecting psychological burden on the informal caregiver of patients with cirrhosis: looking beyond the patient. Journal of Clinical and Experimental Hepatology 2020;10(1):9-16. [DOI: 10.1016/j.jceh.2019.06.002] [PMCID: PMC6995890] [PMID: ] - DOI - PMC - PubMed
Shukla 2011
-
- Shukla S, Mehboob S, Guha S. Comparison of efficacy of rifaximin and non-absorbable disaccharides in management of hepatic encephalopathy: a meta-analysis. Gastroenterology 2011;140(5 Suppl 1):S955. [DOI: 10.1016/S0016-5085(11)63955-3] - DOI
Siddiqui 2021
-
- Siddiqui K, Attria S, Olando M, Lelli F, Maida V, Thabut D. Cost-effectiveness of rifaximin/alpha versus lactulose in preventing recurrent episodes of overt hepatic encephalopathy: a systematic review and meta-analysis. Journal of Hepatology 2021;75(Suppl 2):S356-7.
Soni 2020
Stepanova 2012
Stewart 2007
Tapper 2016
-
- Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A quality improvement initiative reduces 30-day rate of readmission for patients with cirrhosis. Clinical Gastroenterology and Hepatology 2016;14(5):753-9. [DOI: 10.1016/j.cgh.2015.08.041] [PMCID: PMC5394424] [PMID: ] - DOI - PMC - PubMed
Tapper 2019
Tapper 2020
Teasdale 1974
-
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81-4. [PMID: ] - PubMed
Trebicka 2021
van Leeuwen 1988
Victor 1965
-
- Victor M, Adams RD, Cole M. The acquired (non-Wilsonian) type of chronic hepatocerebral degeneration. Medicine 1965;44:345-96. [PMID: ] - PubMed
Wang 2019a
Wang 2019b
-
- Wang W, Yang J, Liu C, Song P, Wang W, Xu H, et al. Norfloxacin, ciprofloxacin, trimethoprim-sulfamethoxazole, and rifaximin for the prevention of spontaneous bacterial peritonitis: a network meta-analysis. Journal of Gastroenterology and Hepatology 2019;31(8):905-10. [DOI: 10.1097/MEG.0000000000001446] [PMID: ] - DOI - PubMed
Weber 1987
-
- Weber FL Jr, Banwell JG, Fresard KM, Cummings JH. Nitrogen in fecal bacterial, fibre, and soluble fractions ofpatients with cirrhosis: effects of lactulose and lactuloseplus neomycin. Journal of Laboratory and Clinical Medicine 1987;110(3):259-63. [PMID: ] - PubMed
Weissenborn 1998
Weissenborn 2001
-
- Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. Journal of Hepatology 2001;34:768-73. [PMID: ] - PubMed
Weissenborn 2019
Wong 2014
Wu 2013
Zacharias 2017
-
- Zacharias HD, Jackson CD, Morgan MY, Olesen SS. Letter: stepwise diagnosis in covert hepatic encephalopathy - critical flicker frequency and MELD-score as a first-step approach. Replication and pitfalls. Alimentary Pharmacology and Therapeutics 21017;45(1):187-9. [DOI: 10.1111/apt.13830] [PMID: ] - DOI - PubMed
Zacharias 2019
-
- Zacharias HD, Zacharias AP, Gluud LL, Morgan MY. Pharmacotherapies that specifically target ammonia for the prevention and treatment of hepatic encephalopathy in adults with cirrhosis. Cochrane Database of Systematic Reviews 2019, Issue 6(6) CD012334. Art. No: CD012334. [DOI: 10.1002/14651858.CD012334.pub2] - DOI - PMC - PubMed
Zhu 2015
-
- Zhu GQ, Shi KQ, Huang S, Wang LR, Lin YQ, Huang GQ, et al. Systematic review with network meta-analysis: the comparative effectiveness and safety of interventions in patients with overt hepatic encephalopathy. Alimentary Pharmacology and Therapeutics 2015;41(7):624-35. [DOI: 10.1111/apt.13122] - DOI - PubMed
Zhu 2016
Zhu 2019
-
- Zuo L, Iv Y, Wang Q, Yin Z, Wang Z, He C, et al. Early-recurrent overt hepatic encephalopathy is associated with reduced survival in cirrhotic patients after transjugular intrahepatic portosystemic shunt creation. Journal of Vascular and Interventional Radiology 2019;30(2):148-52.e2. [DOI: 10.1016/j.jvir.2018.08.023] [PMID: ] - DOI - PubMed
Zhuo 2019
-
- Zhuo GY, Xiang T, Zhang PY, Zhang XD, Luo L, Zhang JM, et al. Effects of rifaximin versus nonabsorbable disaccharides in hepatic encephalopathy. GastroHep 2019;1(1):22-32. [DOI: 10.1002/ygh2.207] - DOI
References to other published versions of this review
Kimer 2015
-
- Kimer N, Krag A, Bendtsen F, Møller S, Gluud LL. Rifaximin for people with hepatic encephalopathy. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD011585. [DOI: 10.1002/14651858.CD011585] - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

