Lumicitabine, an orally administered nucleoside analog, in infants hospitalized with respiratory syncytial virus (RSV) infection: Safety, efficacy, and pharmacokinetic results
- PMID: 37467213
- PMCID: PMC10355467
- DOI: 10.1371/journal.pone.0288271
Lumicitabine, an orally administered nucleoside analog, in infants hospitalized with respiratory syncytial virus (RSV) infection: Safety, efficacy, and pharmacokinetic results
Abstract
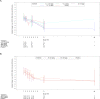
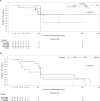
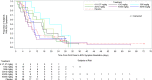
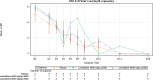
Respiratory syncytial virus (RSV) infection is the leading cause of infant hospitalizations and mortality. Lumicitabine, an oral nucleoside analog was studied for the treatment of RSV. The phase 1b and phase 2b studies reported here assessed the safety, pharmacokinetics, and pharmacodynamics of lumicitabine in infants/neonates hospitalized with RSV. In the phase 1b study, infants (≥1 to ≤12 months) and neonates (<28 days) received a single-ascending or multiple-ascending doses (single loading dose [LD] then 9 maintenance doses [MD] of lumicitabine, or placebo [3:1]). In the phase 2b study, infants/children (28 days to ≤36 months old) received lumicitabine 40/20 mg/kg, 60/40 mg/kg LD/MD twice-daily or placebo (1:1:1) for 5 days. Safety, pharmacokinetics, and efficacy parameters were assessed over 28 days. Lumicitabine was associated with a dose-related increase in the incidence and severity of reversible neutropenia. Plasma levels of ALS-008112, the active nucleoside analog, were dose-proportional with comparable mean exposure levels at the highest doses in both studies. There were no significant differences between the lumicitabine groups and placebo in reducing viral load, time to viral non-detectability, and symptom resolution. No emergent resistance-associated substitutions were observed at the RSV L-gene positions of interest. In summary, lumicitabine was associated with a dose-related increase in the incidence and severity of reversible neutropenia and failed to demonstrate antiviral activity in RSV-infected hospitalized infants. This contrasts with the findings of the previous RSV-A adult challenge study where significant antiviral activity was noted, without incidence of neutropenia. Trial registration ClinicalTrials.gov Identifier: NCT02202356 (phase 1b); NCT03333317 (phase 2b).
Copyright: © 2023 Oey et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Abbie Oey, Matthew McClure, Julian A. Symons, Sushmita Chanda, John Fry, Dymphy Huntjens, and James Witek are current or former employees of Janssen Research & Development and may own stock/stock options in Johnson & Johnson. Patrick Smith works for Certara, a consulting firm in integrated drug development and has directly consulted with a variety of not‐for‐profit global health organizations, biotechnology, and pharmaceutical companies and governments with an interest in medical countermeasures against respiratory virus infections. Kathia Luciani, Michael Fayon, Kulkanya Chokephaibulkit, Rattapon Uppala, Jolanta Bernatoniene, Kenji Furuno, and Thorsten Stanley have no potential conflicts of interest to disclose. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al.. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet. 2017;390:946–58. doi: 10.1016/S0140-6736(17)30938-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

