Nicotinamide enhances natural killer cell function and yields remissions in patients with non-Hodgkin lymphoma
- PMID: 37467318
- PMCID: PMC10859734
- DOI: 10.1126/scitranslmed.ade3341
Nicotinamide enhances natural killer cell function and yields remissions in patients with non-Hodgkin lymphoma
Abstract
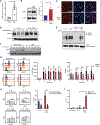
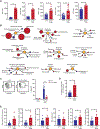
Allogeneic natural killer (NK) cell adoptive transfer has shown the potential to induce remissions in relapsed or refractory leukemias and lymphomas, but strategies to enhance NK cell survival and function are needed to improve clinical efficacy. Here, we demonstrated that NK cells cultured ex vivo with interleukin-15 (IL-15) and nicotinamide (NAM) exhibited stable induction of l-selectin (CD62L), a lymphocyte adhesion molecule important for lymph node homing. High frequencies of CD62L were associated with elevated transcription factor forkhead box O1 (FOXO1), and NAM promoted the stability of FOXO1 by preventing proteasomal degradation. NK cells cultured with NAM exhibited metabolic changes associated with elevated glucose flux and protection against oxidative stress. NK cells incubated with NAM also displayed enhanced cytotoxicity and inflammatory cytokine production and preferentially persisted in xenogeneic adoptive transfer experiments. We also conducted a first-in-human phase 1 clinical trial testing adoptive transfer of NK cells expanded ex vivo with IL-15 and NAM (GDA-201) combined with monoclonal antibodies in patients with relapsed or refractory non-Hodgkin lymphoma (NHL) and multiple myeloma (MM) (NCT03019666). Cellular therapy with GDA-201 and rituximab was well tolerated and yielded an overall response rate of 74% in 19 patients with advanced NHL. Thirteen patients had a complete response, and 1 patient had a partial response. GDA-201 cells were detected for up to 14 days in blood, bone marrow, and tumor tissues and maintained a favorable metabolic profile. The safety and efficacy of GDA-201 in this study support further development as a cancer therapy.
Conflict of interest statement
Figures






References
-
- Narni-Mancinelli E, Ugolini S, Vivier E, Tuning the threshold of natural killer cell responses. Curr. Opin. Immunol. 25, 53–58 (2013). - PubMed
-
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB, Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3057 (2005). - PubMed
-
- Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS, Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 123, 3855–3863 (2014). - PMC - PubMed
-
- Bachanova V, Sarhan D, DeFor TE, Cooley S, Panoskaltsis-Mortari A, Blazar BR, Curtsinger JM, Burns L, Weisdorf DJ, Miller JS, Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol. Immunother. 67, 483–494 (2018). - PMC - PubMed
-
- Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer GN, Lerrer B, Cohen HY, Nagler A, Fibach E, Peled A, Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp. Hematol. 40, 342–355.e1 (2012). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

