d-lactate modulates M2 tumor-associated macrophages and remodels immunosuppressive tumor microenvironment for hepatocellular carcinoma
- PMID: 37467325
- PMCID: PMC10355835
- DOI: 10.1126/sciadv.adg2697
d-lactate modulates M2 tumor-associated macrophages and remodels immunosuppressive tumor microenvironment for hepatocellular carcinoma
Abstract
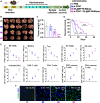
The polarization of tumor-associated macrophages (TAMs) from M2 to M1 phenotype demonstrates great potential for remodeling the immunosuppressive tumor microenvironment (TME) of hepatocellular carcinoma (HCC). d-lactate (DL; a gut microbiome metabolite) acts as an endogenous immunomodulatory agent that enhances Kupffer cells for clearance of pathogens. In this study, the potential of DL for transformation of M2 TAMs to M1 was confirmed, and the mechanisms underlying such polarization were mainly due to the modulation of phosphatidylinositol 3-kinase/protein kinase B pathway. A poly(lactide-co-glycolide) nanoparticle (NP) was used to load DL, and the DL-loaded NP was modified with HCC membrane and M2 macrophage-binding peptide (M2pep), forming a nanoformulation (DL@NP-M-M2pep). DL@NP-M-M2pep transformed M2 TAMs to M1 and remodeled the immunosuppressive TME in HCC mice, promoting the efficacy of anti-CD47 antibody for long-term animal survival. These findings reveal a potential TAM modulatory function of DL and provide a combinatorial strategy for HCC immunotherapy.
Figures




References
-
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, F. Bray, Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). - PubMed
-
- P. J. Trivedi, D. H. Adams, Gut-liver immunity. J. Hepatol. 64, 1187–1189 (2016). - PubMed
-
- F. Heymann, F. Tacke, Immunology in the liver—From homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13, 88–110 (2016). - PubMed
-
- C. N. Jenne, P. Kubes, Immune surveillance by the liver. Nat. Immunol. 14, 996–1006 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

