Semiconducting Polymer Nanoporous Thin Films as a Tool to Regulate Intracellular ROS Balance in Endothelial Cells
- PMID: 37467460
- PMCID: PMC10401575
- DOI: 10.1021/acsami.3c06633
Semiconducting Polymer Nanoporous Thin Films as a Tool to Regulate Intracellular ROS Balance in Endothelial Cells
Abstract
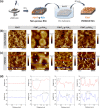
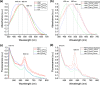
The design of soft and nanometer-scale photoelectrodes able to stimulate and promote the intracellular concentration of reactive oxygen species (ROS) is searched for redox medicine applications. In this work, we show semiconducting polymer porous thin films with an enhanced photoelectrochemical generation of ROS in human umbilical vein endothelial cells (HUVECs). To achieve that aim, we synthesized graft copolymers, made of poly(3-hexylthiophene) (P3HT) and degradable poly(lactic acid) (PLA) segments, P3HT-g-PLA. In a second step, the hydrolysis of sacrificial PLA leads to nanometer-scale porous P3HT thin films. The pore sizes in the nm regime (220-1200 nm) were controlled by the copolymer composition and the structural arrangement of the copolymers during the film formation, as determined by atomic force microscopy (AFM) and transmission electron microscopy (TEM). The porous P3HT thin films showed enhanced photofaradaic behavior, generating a higher concentration of ROS in comparison to non-porous P3HT films, as determined by scanning electrochemical microscopy (SECM) measurements. The exogenous ROS production was able to modulate the intracellular ROS concentration in HUVECs at non-toxic levels, thus affecting the physiological functions of cells. Results presented in this work provide an important step forward in the development of new tools for precise, on-demand, and non-invasive modulation of intracellular ROS species and may be potentially extended to many other physiological or pathological cell models.
Keywords: biophotonics; cell optical modulation; poly(3-hexylthiophene); porous films; reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Adhikari A.; Mondal S.; Chatterjee T.; Das M.; Biswas P.; Ghosh R.; Darbar S.; Alessa H.; Althakafy J. T.; Sayqal A.; et al. Redox nanomedicine ameliorates chronic kidney disease (CKD) by mitochondrial reconditioning in mice. Commun. Biol. 2021, 4, 1013.10.1038/s42003-021-02546-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

