Chemoenzymatic Synthesis of Tenofovir
- PMID: 37467462
- PMCID: PMC10407936
- DOI: 10.1021/acs.joc.3c01005
Chemoenzymatic Synthesis of Tenofovir
Abstract
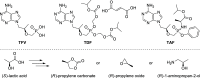
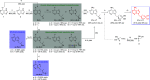
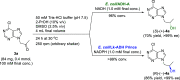
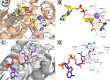
We report on novel chemoenzymatic routes toward tenofovir using low-cost starting materials and commercial or homemade enzyme preparations as biocatalysts. The biocatalytic key step was accomplished either via stereoselective reduction using an alcohol dehydrogenase or via kinetic resolution using a lipase. By employing a suspension of immobilized lipase from Burkholderia cepacia (Amano PS-IM) in a mixture of vinyl acetate and toluene, the desired (R)-ester (99% ee) was obtained on a 500 mg scale (60 mM) in 47% yield. Alternatively, stereoselective reduction of 1-(6-chloro-9H-purin-9-yl) propan-2-one (84 mg, 100 mM) catalyzed by lyophilized E. coli cells harboring recombinant alcohol dehydrogenase (ADH) from Lactobacillus kefir (E. coli/Lk-ADH Prince) allowed one to reach quantitative conversion, 86% yield and excellent optical purity (>99% ee) of the corresponding (R)-alcohol. The key (R)-intermediate was transformed into tenofovir through "one-pot" aminolysis-hydrolysis of (R)-acetate in NH3-saturated methanol, alkylation of the resulting (R)-alcohol with tosylated diethyl(hydroxymethyl) phosphonate, and bromotrimethylsilane (TMSBr)-mediated cleavage of the formed phosphonate ester into the free phosphonic acid. The elaborated enzymatic strategy could be applicable in the asymmetric synthesis of tenofovir prodrug derivatives, including 5'-disoproxil fumarate (TDF, Viread) and 5'-alafenamide (TAF, Vemlidy). The molecular basis of the stereoselectivity of the employed ADHs was revealed by molecular docking studies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Calcaterra A.; D’Acquarica I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. 10.1016/j.jpba.2017.07.008. - DOI - PubMed
- Brooks W. H.; Guida W. C.; Daniel K. G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. 10.2174/156802611795165098. - DOI - PMC - PubMed
-
- Borowiecki P.; Zdun B.; Popow N.; Wiklinska M.; Reiter T.; Kroutil W. Development of a novel chemoenzymatic route to enantiomerically enriched beta-adrenolytic agents. A case study toward propranolol, alprenolol, pindolol, carazolol, moprolol, and metoprolol. RSC Adv. 2022, 12, 22150–22160. 10.1039/D2RA04302E. - DOI - PMC - PubMed
- Borowiecki P.; Rudzka A.; Reiter T.; Kroutil W. Biocatalytic hydrogen-transfer to access enantiomerically pure proxyphylline, xanthinol, and diprophylline. Bioorg. Chem. 2022, 127, 105967.10.1016/j.bioorg.2022.105967. - DOI - PubMed
- Borowiecki P.; Rudzka A.; Reiter T.; Kroutil W. Chemoenzymatic deracemization of lisofylline catalyzed by a (laccase/TEMPO)-alcohol dehydrogenase system. Catal. Sci. Technol. 2022, 12, 4312–4324. 10.1039/D2CY00145D. - DOI
- Zdun B.; Cieśla P.; Kutner J.; Borowiecki P. Expanding Access to Optically Active Non-Steroidal Anti-Inflammatory Drugs via Lipase-Catalyzed KR of Racemic Acids Using Trialkyl Orthoesters as Irreversible Alkoxy Group Donors. Catalysts 2022, 12, 546.10.3390/catal12050546. - DOI
- Borowiecki P.; Zdun B.; Dranka M. Chemoenzymatic enantioselective and stereo-convergent syntheses of lisofylline enantiomers via lipase-catalyzed kinetic resolution and optical inversion approach. Mol. Catal. 2021, 504, 111451.10.1016/j.mcat.2021.111451. - DOI
- Borowiecki P.; Młynek M.; Dranka M. Chemoenzymatic synthesis of enantiomerically enriched diprophylline and xanthinol nicotinate. Bioorg. Chem. 2021, 106, 104448.10.1016/j.bioorg.2020.104448. - DOI - PubMed
- Poterała M.; Dranka M.; Borowiecki P. Chemoenzymatic Preparation of Enantiomerically Enriched (R)-(−)-Mandelic Acid Derivatives: Application in the Synthesis of the Active Agent Pemoline. Eur. J. Org. Chem. 2017, 2017, 2290–2304. 10.1002/ejoc.201700161. - DOI
- Borowiecki P.; Paprocki D.; Dudzik A.; Plenkiewicz J. Chemoenzymatic Synthesis of Proxyphylline Enantiomers. J. Org. Chem. 2016, 81, 380–395. 10.1021/acs.joc.5b01840. - DOI - PubMed
- Borowiecki P.; Paprocki D.; Dranka M. First chemoenzymatic stereodivergent synthesis of both enantiomers of promethazine and ethopropazine. Beilstein J. Org. Chem. 2014, 10, 3038–3055. 10.3762/bjoc.10.322. - DOI - PMC - PubMed
-
- Ray A. S.; Fordyce M. W.; Hitchcock M. J. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antivir. Res. 2016, 125, 63–70. 10.1016/j.antiviral.2015.11.009. - DOI - PubMed
- Amblard F.; Patel D.; Michailidis E.; Coats S. J.; Kasthuri M.; Biteau N.; Tber Z.; Ehteshami M.; Schinazi R. F. HIV nucleoside reverse transcriptase inhibitors. Eur. J. Med. Chem. 2022, 240, 114554.10.1016/j.ejmech.2022.114554. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

