Escape from T-cell-targeting immunotherapies in acute myeloid leukemia
- PMID: 37467496
- PMCID: PMC11251208
- DOI: 10.1182/blood.2023019961
Escape from T-cell-targeting immunotherapies in acute myeloid leukemia
Abstract
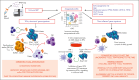
Single-cell and spatial multimodal technologies have propelled discoveries of the solid tumor microenvironment (TME) molecular features and their correlation with clinical response and resistance to immunotherapy. Computational tools are incessantly being developed to characterize tumor-infiltrating immune cells and to model tumor immune escape. These advances have led to substantial research into T-cell hypofunctional states in the TME and their reinvigoration with T-cell-targeting approaches, including checkpoint inhibitors (CPIs). Until recently, we lacked a high-dimensional picture of the acute myeloid leukemia (AML) TME, including compositional and functional differences in immune cells between disease onset and postchemotherapy or posttransplantation relapse, and the dynamic interplay between immune cells and AML blasts at various maturation stages. AML subgroups with heightened interferon gamma (IFN-γ) signaling were shown to derive clinical benefit from CD123×CD3-bispecific dual-affinity retargeting molecules and CPIs, while being less likely to respond to standard-of-care cytotoxic chemotherapy. In this review, we first highlight recent progress into deciphering immune effector states in AML (including T-cell exhaustion and senescence), oncogenic signaling mechanisms that could reduce the susceptibility of AML cells to T-cell-mediated killing, and the dichotomous roles of type I and II IFN in antitumor immunity. In the second part, we discuss how this knowledge could be translated into opportunities to manipulate the AML TME with the aim to overcome resistance to CPIs and other T-cell immunotherapies, building on recent success stories in the solid tumor field, and we provide an outlook for the future.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.R. reports institutional research grants from MacroGenics Inc (Rockville, MD), Kura Oncology (San Diego, CA), and Wugen (St Louis, MO) outside the submitted work; and has a patent (Bispecific CD123×CD3 Diabodies for the Treatment of Hematological Malignancies [Davidson JK, Church S, Rutella S; US20210395374A1]) assigned to Nottingham Trent University, Nottingham, United Kingdom. J.V. declares no competing financial interests.
Figures

References
-
- Sharma P, Goswami S, Raychaudhuri D, et al. Immune checkpoint therapy-current perspectives and future directions. Cell. 2023;186(8):1652–1669. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

