Learning without contingencies: A loss of synergy between memory and reward circuits in schizophrenia
- PMID: 37467677
- PMCID: PMC10521382
- DOI: 10.1016/j.schres.2023.06.004
Learning without contingencies: A loss of synergy between memory and reward circuits in schizophrenia
Abstract
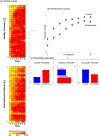
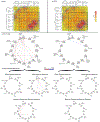
Motivational deficits in schizophrenia may interact with foundational cognitive processes including learning and memory to induce impaired cognitive proficiency. If such a loss of synergy exists, it is likely to be underpinned by a loss of synchrony between the brains learning and reward sub-networks. Moreover, this loss should be observed even during tasks devoid of explicit reward contingencies given that such tasks are better models of real world performance than those with artificial contingencies. Here we applied undirected functional connectivity (uFC) analyses to fMRI data acquired while participants engaged in an associative learning task without contingencies or feedback. uFC was estimated and inter-group differences (between schizophrenia patients and controls, n = 54 total, n = 28 patients) were assessed within and between reward (VTA and NAcc) and learning/memory (Basal Ganglia, DPFC, Hippocampus, Parahippocampus, Occipital Lobe) sub-networks. The task paradigm itself alternated between Encoding, Consolidation, and Retrieval conditions, and uFC differences were quantified for each of the conditions. Significantly reduced uFC dominated the connectivity profiles of patients across all conditions. More pertinent to our motivations, these reductions were observed within and across classes of sub-networks (reward-related and learning/memory related). We suggest that disrupted functional connectivity between reward and learning sub-networks may drive many of the performance deficits that characterize schizophrenia. Thus, cognitive deficits in schizophrenia may in fact be underpinned by a loss of synergy between reward-sensitivity and cognitive processes.
Keywords: Functional connectivity; Reward and learning; Schizophrenia; fMRI.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts of interest to report.
Figures





References
-
- Achim AM, Bertrand MC, Sutton H, Montoya A, Czechowska Y, Malla AK, Joober R, Pruessner JC, Lepage M, 2007. Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Archives of general psychiatry 64(9), 999–1014. - PubMed
-
- Addington J, Addington D, 1998. Visual attention and symptoms in schizophrenia: a 1-year follow-up. Schizophrenia research 34(1–2), 95–99. - PubMed
-
- Alberini CM, LeDoux JE, 2013. Memory reconsolidation. Current Biology 23(17), R746–R750. - PubMed
-
- Amodio A, Quarantelli M, Mucci A, Prinster A, Soricelli A, Vignapiano A, Giordano GM, Merlotti E, Nicita A, Galderisi S, 2018. Avolition-Apathy and White Matter Connectivity in Schizophrenia: Reduced Fractional Anisotropy Between Amygdala and Insular Cortex. Clin EEG Neurosci 49(1), 55–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

