The NLR member CIITA: Master controller of adaptive and intrinsic immunity and unexpected tool in cancer immunotherapy
- PMID: 37467968
- PMCID: PMC10505679
- DOI: 10.1016/j.bj.2023.100631
The NLR member CIITA: Master controller of adaptive and intrinsic immunity and unexpected tool in cancer immunotherapy
Abstract
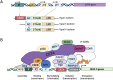
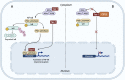
Human nucleotide-binding oligomerization domain (NOD)-like receptors (NLR) include a large family of proteins that have important functions in basic physio-pathological processes like inflammation, cell death and regulation of transcription of key molecules for the homeostasis of the immune system. They are all characterized by a common backbone structure (the STAND ATPase module consisting in a nucleotide-binding domain (NBD), an helical domain 1 (HD1) and a winged helix domain (WHD), used by both prokaryotes and eukaryotes as defense mechanism. In this review, we will focus on the MHC class II transactivator (CIITA), the master regulator of MHC class II (MHC-II) gene expression and the founding member of NLR. Although a consistent part of the described NLR family components is often recalled as innate or intrinsic immune sensors, CIITA in fact occupies a special place as a unique example of regulator of both intrinsic and adaptive immunity. The description of the discovery of CIITA and the genetic and molecular characterization of its expression will be followed by the most recent studies that have unveiled this dual role of CIITA, key molecule in intrinsic immunity as restriction factor for human retroviruses and precious tool to induce the expression of MHC-II molecules in cancer cells, rendering them potent surrogate antigen presenting cells (APC) for their own tumor antigens.
Keywords: CIITA; Cancer; HTLV-1; NLR; Restriction factors.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. - PubMed
-
- Leipe D.D., Koonin E.V., Aravind L. STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. J Mol Biol. 2004;343(1):1–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

