Radiation nephropathy: Mechanisms of injury and recovery in a murine model
- PMID: 37468066
- PMCID: PMC11648365
- DOI: 10.1016/j.radonc.2023.109813
Radiation nephropathy: Mechanisms of injury and recovery in a murine model
Abstract
Background: Radiation nephropathy (RN) can be a severe late complication for patients treated with radiotherapy (RT) targeting abdominal and paraspinal tumors. Recent studies investigating the mechanisms of RT-mediated injury in the kidney have demonstrated that RT disrupts the cellular integrity of renal podocytes leading to cell death and loss of renal function.
Aim: To determine if RT-induced renal dysfunction is associated with alterations in podocyte and glomerular function, and whether RT-induced podocyte alterations were associated with changes in the glomerular basement membrane (GBM).
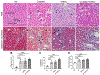
Methods: C57BL/6 mice were treated with focal bilateral X-irradiation using a single dose (SD) of 4 Gy, 10 Gy, or 14 Gy or fractionated dosing (FD) of 5x6Gy or 24x2Gy. Then, 10-40 weeks after RT parameters of renal function were measured, along with glomerular filtration rate (GFR) and glomerular histology, as well as ultrastructural changes in GBM by transmission electron microscopy.
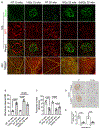
Results: RT treatment resulted in persistent changes in renal function beginning at 10 weeks with little recovery up to 40 weeks post RT. Dose dependent changes were seen with increasing SD but no functional sparing was evident after FD. RT-induced loss of renal function was associated with expansion of the GBM and significant increases in foot process width, and associated with significant reduction in GFR, podocyte loss, and renal fibrosis.
Conclusion: For the first time, these data show that expansion of the GBM is one consequence of radiation injury, and disarrangement of the GBM might be associated with the death of podocytes. These data shed new light on the role podocyte injury and GBM in RT-induced renal dysfunction.
Keywords: Glomerular basement membrane; Ionizing radiation; Podocyte foot process; Podocytes; Radiation therapy.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




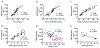

References
-
- De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F. Radiotherapy toxicity. Nat Rev Dis Primers 2019;5:13. - PubMed
-
- Dawson LA, Kavanagh BD, Paulino AC, Das SK, Miften M, Li XA, et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys 2010;76:S108–15. - PubMed
-
- Cassady JR. Clinical radiation nephropathy. Int J Radiat Oncol Biol Phys 1995;31:1249–56. - PubMed
-
- Tarbell NJ, Guinan EC, Chin L, Mauch P, Weinstein HJ. Renal insufficiency after total body irradiation for pediatric bone marrow transplantation. Radiother Oncol 1990;18:139–42. - PubMed
-
- Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

