Role of Human Epicardial Adipose Tissue-Derived miR-92a-3p in Myocardial Redox State
- PMID: 37468187
- PMCID: PMC10368522
- DOI: 10.1016/j.jacc.2023.05.031
Role of Human Epicardial Adipose Tissue-Derived miR-92a-3p in Myocardial Redox State
Abstract
Background: Visceral obesity is directly linked to increased cardiovascular risk, including heart failure.
Objectives: This study explored the ability of human epicardial adipose tissue (EAT)-derived microRNAs (miRNAs) to regulate the myocardial redox state and clinical outcomes.
Methods: This study screened for miRNAs expressed and released from human EAT and tested for correlations with the redox state in the adjacent myocardium in paired EAT/atrial biopsy specimens from patients undergoing cardiac surgery. Three miRNAs were then tested for causality in an in vitro model of cardiomyocytes. At a clinical level, causality/directionality were tested using genome-wide association screening, and the underlying mechanisms were explored using human biopsy specimens, as well as overexpression of the candidate miRNAs and their targets in vitro and in vivo using a transgenic mouse model. The final prognostic value of the discovered targets was tested in patients undergoing cardiac surgery, followed up for a median of 8 years.
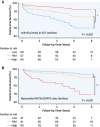
Results: EAT miR-92a-3p was related to lower oxidative stress in human myocardium, a finding confirmed by using genetic regulators of miR-92a-3p in the human heart and EAT. miR-92a-3p reduced nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase-derived superoxide (O2.-) by targeting myocardial expression of WNT5A, which regulated Rac1-dependent activation of NADPH oxidases. Finally, high miR-92a-3p levels in EAT were independently related with lower risk of adverse cardiovascular events.
Conclusions: EAT-derived miRNAs exert paracrine effects on the human heart. Indeed miR-92a-3p suppresses the wingless-type MMTV integration site family, member 5a/Rac1/NADPH oxidase axis and improves the myocardial redox state. EAT-derived miR-92a-3p is related to improved clinical outcomes and is a rational therapeutic target for the prevention and treatment of obesity-related heart disease.
Keywords: Wnt5a signaling; epicardial adipose tissue; microRNAs; myocardial NADPH oxidase activity; myocardial oxidative stress.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Funding Support and Author Disclosures This study was supported by a Marie Skłodowska-Curie Early Stage Researcher fellowship to Dr Carena; the CATCH ME (Characterizing Atrial fibrillation by Translating its Causes into Health Modifiers in the Elderly) consortium (grant number 633196); the British Heart Foundation (FS/16/15/32047, RG/F/21/110040 and CH/F/21/90009 to Dr Antoniades; CH/16/1/32013 to Dr Channon; CH/12/3/29609 to Dr Casadei); Oxford BHF Centre of Research Excellence RE/18/3/34214, the Oxford NIHR Biomedical Research Centre, the National Institute for Health Research Oxford Biomedical Research Centre, and the Novo Nordisk Foundation (NNF15CC0018486) to Dr Antoniades. Dr Antoniades has had consultancy agreements with Mitsubishi Tanabe and Silence Therapeutics; has received grants from Sanofi and Novo Nordisk; is the Chair of the British Atherosclerosis Society; and has received honoraria from Amarin and Covance. Drs Antoniades and Channon are founders, shareholders, and directors of Caristo Diagnostics. Dr Casadei is the past president of the European Society of Cardiology. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures








Comment in
-
Epicardial Adipose-Derived miR92a-3p: Stress Reduction Therapy?J Am Coll Cardiol. 2023 Jul 25;82(4):333-335. doi: 10.1016/j.jacc.2023.05.030. J Am Coll Cardiol. 2023. PMID: 37468188 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- CH/16/1/32013/BHF_/British Heart Foundation/United Kingdom
- RG/F/22/110085/BHF_/British Heart Foundation/United Kingdom
- RG/F/21/110040/BHF_/British Heart Foundation/United Kingdom
- CH/F/21/90009/BHF_/British Heart Foundation/United Kingdom
- RE/18/3/34214/BHF_/British Heart Foundation/United Kingdom
- RG/17/10/32859/BHF_/British Heart Foundation/United Kingdom
- TG/19/2/34831/BHF_/British Heart Foundation/United Kingdom
- FS/16/15/32047/BHF_/British Heart Foundation/United Kingdom
- CH/12/3/29609/BHF_/British Heart Foundation/United Kingdom
- DH_/Department of Health/United Kingdom
- RG/16/12/32451/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Research Materials

