Association of Very Early Treatment Initiation With the Risk of Long-term Disability in Patients With a First Demyelinating Event
- PMID: 37468284
- PMCID: PMC10558169
- DOI: 10.1212/WNL.0000000000207664
Association of Very Early Treatment Initiation With the Risk of Long-term Disability in Patients With a First Demyelinating Event
Abstract
Background and objectives: Early treatment is associated with better long-term outcomes in patients with a first demyelinating event and early multiple sclerosis (MS). However, magnetic resonance (MR) findings are not usually integrated to construct propensity scores (PSs) when evaluating outcomes. We assessed the association of receiving very early treatment with the risk of long-term disability including an MR score (MRS) in patients with a first demyelinating event.
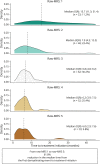
Methods: We included 580 patients with a first demyelinating event prospectively collected between 1994 and 2021, who received at least 1 disease-modifying drug (DMD). Patients were classified into tertiles according to the cohort's distribution of the time from the first demyelinating event to the first DMD: first tertile (FT) or very early treatment (6 months; n = 194), second tertile (6.1-16 months, n = 192), and third tertile (TT) (16.1 months, n = 194). A 5-point MRS was built according to the sum of the following indicators: ≥9 brain lesions (1 point); ≥1 infratentorial lesion (1 point); ≥1 spinal cord (SC) lesion (1 point); ≥1 contrast-enhancing (CE) brain lesion (1 point); and ≥1 CE SC lesion (1 point). PS based on covariates and the MRS was computed for each of the outcomes. Inverse PS-weighted Cox and linear regression models assessed the risk of different outcomes between tertile groups. Finally, to confirm the role of MR in treatment decision, we studied the time elapsed from the first demyelinating event to treatment initiation according to the MRS in all patients with radiologic available information, renamed as raw-MRS.
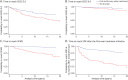
Results: Very early treatment decreased the risk of reaching Expanded Disability Status Scale 3.0 (hazard ratio [HR] 0.55, 95% CI 0.32-0.97), secondary progressive MS (HR 0.40, 95% CI 0.19-0.85), and sustained disease progression at 12 months after treatment initiation (HR 0.50, 95% CI 0.29-0.84), when compared with patients from the TT group. Patients from the FT group had a lower disability progression rate (β estimate -0.009, 95% CI -0.016 to -0.002) and a lower severe disability measured by the Patient-Determined Disease Step (β estimate -0.52, 95% CI -0.91 to -0.13) than the TT group. Finally, there was a 62.4% reduction in the median time between the first demyelinating event and the first-ever treatment initiation from patients displaying a raw-MRS 1 to patients with a raw-MRS 5.
Discussion: Using PS models with and without MRS, we showed that treatment initiation at very early stages is associated with a reduction in the risk of long-term disability accrual in patients with a first demyelinating event.
Classification of evidence: This study provides Class III evidence that earlier treatment of patients with MS presenting with a first demyelinating event is associated with improved clinical outcomes.
© 2023 American Academy of Neurology.
Conflict of interest statement
A. Cobo-Calvo has received grant from Instituto de Salud Carlos III, Spain, JR19/00007. C. Tur has is currently being funded by a Junior Leader La Caixa Fellowship (fellowship code is LCF/BQ/PI20/11760008), awarded by “la Caixa” Foundation (ID 100010434). She has also received the 2021 Merck's Award for the Investigation in MS, awarded by Fundación Merck Salud (Spain) and a grant awarded by the Instituto de Salud Carlos III (ISCIII), Ministerio de Ciencia e Innovación de España (PI21/01860). In 2015, she received an ECTRIMS Postdoctoral Research Fellowship and has received funding from the UK MS Society. She has also received honoraria from Roche and Novartis and is a steering committee member of the O'HAND trial and of the Consensus group on Follow-on DMTs. S. Otero-Romero has received speaking and consulting honoraria from Genzyme, Biogen-Idec, Novartis, Excemed, Bayer HealthCare Pharmaceuticals, BiogenGSK, and MSD and research support from Novartis. P. Carbonell-Mirabent has received support from traveling from Biogen, and his yearly salary is supported by a grant from Biogen to Fundacio privada Cemcat for statistical analysis. M. Ruiz and A. Papolla report no disclosures. J. Villacieros Alvarez has received grant from Instituto de Salud Carlos III, Spain; + FI21/00282. A. Vidal-Jordana reports no disclosures. G. Arrambide has received compensation for consulting services or participation in advisory boards from Sanofi, Merck, Roche, and Horizon Therapeutics; travel support for scientific meetings from Novartis, Roche, and ECTRIMS; and speaking honoraria from Sanofi, Merck, and Roche. Arrambide is a member of the executive committee of the International Women in Multiple Sclerosis (iWiMS) network and of the European Biomarkers in MS (BioMS-eu) Network. J. Castilló, I. Galan, M. Rodríguez Barranco, and L.S. Midaglia report no disclosures relevant to the manuscript. C. Nos has received consultation honoraria from Roche. B. Rodriguez Acevedo reports no disclosures relevant to the manuscript. A. Zabalza de Torres has received travel expenses for scientific meetings from Biogen-Idec and Novartis, speaking honoraria from Eisai, and a study grant from Novartis. N. Mongay has a predoctoral grant Rio Hortega from Instituto Carlos III (CM21/00018). J. Rio has received speaking honoraria and personal compensation for participating on Advisory Boards from Biogen-Idec, Genzyme, Merck-Serono, Novartis, Teva, Roche, Janssen, and Sanofi-Aventis. M. Comabella reports no disclosures relevant to the manuscript. C. Auger reports no disclosures relevant to the manuscript. J. Sastre-Garriga has received compensation for consulting services and speaking honoraria from Almirall, Bayer, Biogen, Celgene, Sanofi, Merck, Novartis, Roche, Bial, Biopass, and Teva, is member of the editorial committee of Multiple Sclerosis Journal, and director of Revista de Neurología. A. Rovira serves/served on scientific advisory boards for Novartis, Sanofi-Genzyme, SyntheticMR, Bayer, Roche, Biogen, Icometrix, and OLEA Medical and has received speaker honoraria from Bayer, Sanofi-Genzyme, Bracco, Merck-Serono, Teva Pharmaceutical Industries Ltd., Novartis, Roche, and Biogen. M. Tintore has received compensation for consulting services and speaking honoraria from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Viela-Bio, and Teva Pharmaceuticals. M. Tintore is coeditor of Multiple Sclerosis Journal—Experimental, Translational and Clinical. X. Montalban has received speaking honoraria and travel expenses for scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past 3 years with Biogen Idec, Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceuticals, Roche, Celgene, Actelion, Mylan, and BMS. Go to
Figures
Comment in
-
Early Treatment for Multiple Sclerosis: Time Is Brain.Neurology. 2023 Sep 26;101(13):549-550. doi: 10.1212/WNL.0000000000207754. Epub 2023 Jul 19. Neurology. 2023. PMID: 37468283 No abstract available.
References
-
- Comi G, Martinelli V, Rodegher M, et al. . Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374(9700):1503-1511. doi:10.1016/s0140-6736(09)61259-9 - DOI - PubMed
-
- Comi G, de Stefano N, Freedman MS, et al. . Subcutaneous interferon β-1a in the treatment of clinically isolated syndromes: 3-year and 5-year results of the phase III dosing frequency-blind multicentre REFLEXION study. J Neurol Neurosurg Psychiatry. 2017;88(4):285-294. doi:10.1136/jnnp-2016-314843 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
