Endometrial receptivity in women of advanced age: an underrated factor in infertility
- PMID: 37468438
- PMCID: PMC10628506
- DOI: 10.1093/humupd/dmad019
Endometrial receptivity in women of advanced age: an underrated factor in infertility
Abstract
Background: Modern lifestyle has led to an increase in the age at conception. Advanced age is one of the critical risk factors for female-related infertility. It is well known that maternal age positively correlates with the deterioration of oocyte quality and chromosomal abnormalities in oocytes and embryos. The effect of age on endometrial function may be an equally important factor influencing implantation rate, pregnancy rate, and overall female fertility. However, there are only a few published studies on this topic, suggesting that this area has been under-explored. Improving our knowledge of endometrial aging from the biological (cellular, molecular, histological) and clinical perspectives would broaden our understanding of the risks of age-related female infertility.
Objective and rationale: The objective of this narrative review is to critically evaluate the existing literature on endometrial aging with a focus on synthesizing the evidence for the impact of endometrial aging on conception and pregnancy success. This would provide insights into existing gaps in the clinical application of research findings and promote the development of treatment options in this field.
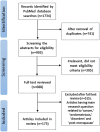
Search methods: The review was prepared using PubMed (Medline) until February 2023 with the keywords such as 'endometrial aging', 'receptivity', 'decidualization', 'hormone', 'senescence', 'cellular', 'molecular', 'methylation', 'biological age', 'epigenetic', 'oocyte recipient', 'oocyte donation', 'embryo transfer', and 'pregnancy rate'. Articles in a language other than English were excluded.
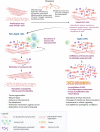
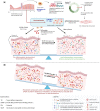
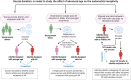
Outcomes: In the aging endometrium, alterations occur at the molecular, cellular, and histological levels suggesting that aging has a negative effect on endometrial biology and may impair endometrial receptivity. Additionally, advanced age influences cellular senescence, which plays an important role during the initial phase of implantation and is a major obstacle in the development of suitable senolytic agents for endometrial aging. Aging is also accountable for chronic conditions associated with inflammaging, which eventually can lead to increased pro-inflammation and tissue fibrosis. Furthermore, advanced age influences epigenetic regulation in the endometrium, thus altering the relation between its epigenetic and chronological age. The studies in oocyte donation cycles to determine the effect of age on endometrial receptivity with respect to the rates of implantation, clinical pregnancy, miscarriage, and live birth have revealed contradictory inferences indicating the need for future research on the mechanisms and corresponding causal effects of women's age on endometrial receptivity.
Wider implications: Increasing age can be accountable for female infertility and IVF failures. Based on the complied observations and synthesized conclusions in this review, advanced age has been shown to have a negative impact on endometrial functioning. This information can provide recommendations for future research focusing on molecular mechanisms of age-related cellular senescence, cellular composition, and transcriptomic changes in relation to endometrial aging. Additionally, further prospective research is needed to explore newly emerging therapeutic options, such as the senolytic agents that can target endometrial aging without affecting decidualization. Moreover, clinical trial protocols, focusing on oocyte donation cycles, would be beneficial in understanding the direct clinical implications of endometrial aging on pregnancy outcomes.
Keywords: cellular senescence; decidualization; endometrial aging; endometrial receptivity; epigenetics; oocyte donation.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures

Similar articles
-
The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions.Hum Reprod Update. 2022 Feb 28;28(2):172-189. doi: 10.1093/humupd/dmab038. Hum Reprod Update. 2022. PMID: 34918084 Free PMC article. Review.
-
Epigenetic clocks provide clues to the mystery of uterine ageing.Hum Reprod Update. 2023 May 2;29(3):259-271. doi: 10.1093/humupd/dmac042. Hum Reprod Update. 2023. PMID: 36515535 Review.
-
Lipid metabolism and endometrial receptivity.Hum Reprod Update. 2022 Nov 2;28(6):858-889. doi: 10.1093/humupd/dmac026. Hum Reprod Update. 2022. PMID: 35639910 Review.
-
Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis.Hum Reprod Update. 2019 Mar 1;25(2):202-223. doi: 10.1093/humupd/dmy044. Hum Reprod Update. 2019. PMID: 30624659
-
The impact of intentional endometrial injury on reproductive outcomes: a systematic review and meta-analysis.Hum Reprod Update. 2019 Jan 1;25(1):95-113. doi: 10.1093/humupd/dmy034. Hum Reprod Update. 2019. PMID: 30388238
Cited by
-
A Successful Pregnancy Outcome Using Laser-Assisted Hatching and Platelet-Rich Plasma Perfusion in Advanced Maternal and Paternal Age: A Case Report.Cureus. 2024 Jul 5;16(7):e63926. doi: 10.7759/cureus.63926. eCollection 2024 Jul. Cureus. 2024. PMID: 39105032 Free PMC article.
-
Required number of blastocysts transferred, and oocytes retrieved to optimize live and cumulative live birth rates in the first complete cycle of IVF for autologous and donated oocytes.Arch Gynecol Obstet. 2024 Nov;310(5):2681-2690. doi: 10.1007/s00404-024-07712-x. Epub 2024 Sep 4. Arch Gynecol Obstet. 2024. PMID: 39231831
-
Analysis of factors influencing clinical pregnancy rates in frozen-thawed embryo transfer cycles.Front Endocrinol (Lausanne). 2025 Jun 25;16:1551530. doi: 10.3389/fendo.2025.1551530. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40636712 Free PMC article.
-
The activation of cGAS-STING pathway causes abnormal uterine receptivity in aged mice.Aging Cell. 2024 Nov;23(11):e14303. doi: 10.1111/acel.14303. Epub 2024 Aug 7. Aging Cell. 2024. PMID: 39113346 Free PMC article.
-
Effect of prior anti-tuberculosis treatment on assisted reproductive outcomes in infertile women: a retrospective cohort study.BMJ Open. 2025 Jul 18;15(7):e098692. doi: 10.1136/bmjopen-2024-098692. BMJ Open. 2025. PMID: 40681192 Free PMC article.
References
-
- Abdalla HI, Baber R, Kirkland A, Leonard T, Power M, Studd JWW. A report on 100 cycles of oocyte donation; factors affecting the outcome. Hum Reprod 1990;5:1018–1022. - PubMed
-
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update 2006;12:731–746. - PubMed
-
- Balmaceda JP, Bernardini L, Ciuffardi I, Felix C, Ord T, Sueldo CE, Asch RH. Oocyte donation in humans: a model to study the effect of age on embryo implantation rate. Hum Reprod 1994;9:2160–2163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

