Vaccine adjuvants: mechanisms and platforms
- PMID: 37468460
- PMCID: PMC10356842
- DOI: 10.1038/s41392-023-01557-7
Vaccine adjuvants: mechanisms and platforms
Abstract
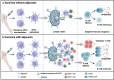
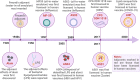
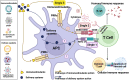
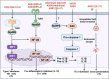
Adjuvants are indispensable components of vaccines. Despite being widely used in vaccines, their action mechanisms are not yet clear. With a greater understanding of the mechanisms by which the innate immune response controls the antigen-specific response, the adjuvants' action mechanisms are beginning to be elucidated. Adjuvants can be categorized as immunostimulants and delivery systems. Immunostimulants are danger signal molecules that lead to the maturation and activation of antigen-presenting cells (APCs) by targeting Toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) to promote the production of antigen signals and co-stimulatory signals, which in turn enhance the adaptive immune responses. On the other hand, delivery systems are carrier materials that facilitate antigen presentation by prolonging the bioavailability of the loaded antigens, as well as targeting antigens to lymph nodes or APCs. The adjuvants' action mechanisms are systematically summarized at the beginning of this review. This is followed by an introduction of the mechanisms, properties, and progress of classical vaccine adjuvants. Furthermore, since some of the adjuvants under investigation exhibit greater immune activation potency than classical adjuvants, which could compensate for the deficiencies of classical adjuvants, a summary of the adjuvant platforms under investigation is subsequently presented. Notably, we highlight the different action mechanisms and immunological properties of these adjuvant platforms, which will provide a wide range of options for the rational design of different vaccines. On this basis, this review points out the development prospects of vaccine adjuvants and the problems that should be paid attention to in the future.
© 2023. The Author(s).
Conflict of interest statement
All authors declare no competing interests. Although Yuquan Wei is the editor-in-chief of “Signal Transduction and Targeted Therapy”, he has not been involved in the process of the manuscript handling.
Figures


References
-
- Glenny A, Pope C, Waddington H, Wallace U. Immunological notes. xvii–xxiv. J. Pathol. Bacteriol. 1926;29:31–40. doi: 10.1002/path.1700290106. - DOI
-
- Freund J, McDermott K. Sensitization to horse serum by means of adjuvants. Proc. Soc. Exp. Biol. Med. 1942;49:548–553. doi: 10.3181/00379727-49-13625. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

