The muscle-enriched myokine Musclin impairs beige fat thermogenesis and systemic energy homeostasis via Tfr1/PKA signaling in male mice
- PMID: 37468484
- PMCID: PMC10356794
- DOI: 10.1038/s41467-023-39710-z
The muscle-enriched myokine Musclin impairs beige fat thermogenesis and systemic energy homeostasis via Tfr1/PKA signaling in male mice
Abstract
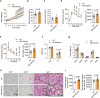
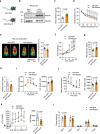
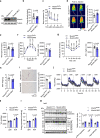
Skeletal muscle and thermogenic adipose tissue are both critical for the maintenance of body temperature in mammals. However, whether these two tissues are interconnected to modulate thermogenesis and metabolic homeostasis in response to thermal stress remains inconclusive. Here, we report that human and mouse obesity is associated with elevated Musclin levels in both muscle and circulation. Intriguingly, muscle expression of Musclin is markedly increased or decreased when the male mice are housed in thermoneutral or chronic cool conditions, respectively. Beige fat is then identified as the primary site of Musclin action. Muscle-transgenic or AAV-mediated overexpression of Musclin attenuates beige fat thermogenesis, thereby exacerbating diet-induced obesity and metabolic disorders in male mice. Conversely, Musclin inactivation by muscle-specific ablation or neutralizing antibody treatment promotes beige fat thermogenesis and improves metabolic homeostasis in male mice. Mechanistically, Musclin binds to transferrin receptor 1 (Tfr1) and antagonizes Tfr1-mediated cAMP/PKA-dependent thermogenic induction in beige adipocytes. This work defines the temperature-sensitive myokine Musclin as a negative regulator of adipose thermogenesis that exacerbates the deterioration of metabolic health in obese male mice and thus provides a framework for the therapeutic targeting of this endocrine pathway.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

