Nucleus reuniens transiently synchronizes memory networks at beta frequencies
- PMID: 37468487
- PMCID: PMC10356781
- DOI: 10.1038/s41467-023-40044-z
Nucleus reuniens transiently synchronizes memory networks at beta frequencies
Abstract
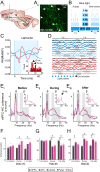
Episodic memory-based decision-making requires top-down medial prefrontal cortex and hippocampal interactions. This integrated prefrontal-hippocampal memory state is thought to be organized by synchronized network oscillations and mediated by connectivity with the thalamic nucleus reuniens (RE). Whether and how the RE synchronizes prefrontal-hippocampal networks in memory, however, remains unknown. Here, we recorded local field potentials from the prefrontal-RE-hippocampal network while rats engaged in a nonspatial sequence memory task, thereby isolating memory-related activity from running-related oscillations. We found that synchronous prefrontal-hippocampal beta bursts (15-30 Hz) dominated during memory trials, whereas synchronous theta activity (6-12 Hz) dominated during non-memory-related running. Moreover, RE beta activity appeared first, followed by prefrontal and hippocampal synchronized beta, suggesting that prefrontal-hippocampal beta could be driven by the RE. To test whether the RE is capable of driving prefrontal-hippocampal beta synchrony, we used an optogenetic approach (retroAAV-ChR2). RE activation induced prefrontal-hippocampal beta coherence and reduced theta coherence, matching the observed memory-driven network state in the sequence task. These findings are the first to demonstrate that the RE contributes to memory by driving transient synchronized beta in the prefrontal-hippocampal system, thereby facilitating interactions that underlie memory-based decision-making.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

