Activation of SARS-CoV-2 by trypsin-like proteases in the clinical specimens of patients with COVID-19
- PMID: 37468582
- PMCID: PMC10356862
- DOI: 10.1038/s41598-023-38757-8
Activation of SARS-CoV-2 by trypsin-like proteases in the clinical specimens of patients with COVID-19
Abstract
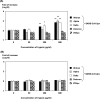
SARS-CoV-2 enters host cells through the angiotensin converting enzyme 2 (ACE2) receptor and/or transmembrane protease, serine 2 (TMPRSS2). In this study, we investigated whether proteases increased SARS-CoV-2 infectivity using pseudotyped viruses and clinical specimens from patients with COVID-19. First, we investigated how trypsin increased infectivity using the pseudotyped virus. Our findings revealed that trypsin increased infectivity after the virus was adsorbed on the cells, but no increase in infectivity was observed when the virus was treated with trypsin. We examined the effect of trypsin on SARS-CoV-2 infection in clinical specimens and found that the infectivity of the SARS-CoV-2 delta variant increased 36,000-fold after trypsin treatment. By contrast, the infectivity of SARS-CoV-2 omicron variant increased to less than 20-fold in the clinical specimens. Finally, using five clinical specimens containing delta variants, enhancement of viral infectivity was evaluated in the presence of the culture supernatant of several anaerobic bacteria. As a result, viral infectivities of all the clinical specimens containing culture supernatants of Fusobacterium necrophorum were significantly increased from several- to tenfold. Because SARS-CoV-2 infectivity increases in the oral cavity, which may contain anaerobic bacteria, keeping the oral cavities clean may help prevent SARS-CoV-2 infection.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- WHO. Coronavirus (COVID-19) Dashboard. Accessed 28 June 2023.
-
- Kido H, Murakami M, Oba K, Chen Y, Towatari T. Cellular proteinases trigger the infectivity of the influenza A and Sendai viruses. Mol. Cells. 1999;9:235–244. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

