A cell-type-specific error-correction signal in the posterior parietal cortex
- PMID: 37468637
- PMCID: PMC10412446
- DOI: 10.1038/s41586-023-06357-1
A cell-type-specific error-correction signal in the posterior parietal cortex
Abstract
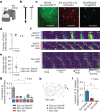
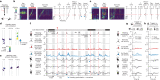
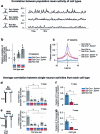
Neurons in the posterior parietal cortex contribute to the execution of goal-directed navigation1 and other decision-making tasks2-4. Although molecular studies have catalogued more than 50 cortical cell types5, it remains unclear what distinct functions they have in this area. Here we identified a molecularly defined subset of somatostatin (Sst) inhibitory neurons that, in the mouse posterior parietal cortex, carry a cell-type-specific error-correction signal for navigation. We obtained repeatable experimental access to these cells using an adeno-associated virus in which gene expression is driven by an enhancer that functions specifically in a subset of Sst cells6. We found that during goal-directed navigation in a virtual environment, this subset of Sst neurons activates in a synchronous pattern that is distinct from the activity of surrounding neurons, including other Sst neurons. Using in vivo two-photon photostimulation and ex vivo paired patch-clamp recordings, we show that nearby cells of this Sst subtype excite each other through gap junctions, revealing a self-excitation circuit motif that contributes to the synchronous activity of this cell type. These cells selectively activate as mice execute course corrections for deviations in their virtual heading during navigation towards a reward location, for both self-induced and experimentally induced deviations. We propose that this subtype of Sst neurons provides a self-reinforcing and cell-type-specific error-correction signal in the posterior parietal cortex that may help with the execution and learning of accurate goal-directed navigation trajectories.
© 2023. The Author(s).
Conflict of interest statement
S.H. is a member of the scientific advisory board and M.E.G. is a consultant for Apertura Gene Therapy. The other authors declare no competing interests.
Figures















References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

