Protrudin-mediated ER-endosome contact sites promote phagocytosis
- PMID: 37468729
- PMCID: PMC10356898
- DOI: 10.1007/s00018-023-04862-0
Protrudin-mediated ER-endosome contact sites promote phagocytosis
Abstract
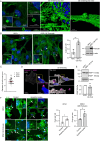
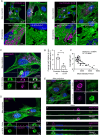
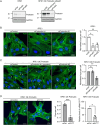
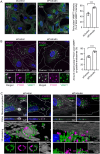
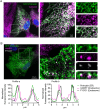
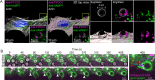
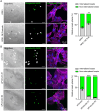
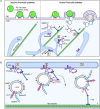
During phagocytosis, endosomes both contribute with membrane to forming phagosomes and promote phagosome maturation. However, how these vesicles are delivered to the phagocytic cup and the phagosome has been unknown. Here, we show that Protrudin-mediated endoplasmic reticulum (ER)-endosome contact sites facilitate anterograde translocation of FYCO1 and VAMP7-positive late endosomes and lysosomes (LELys) to forming phagocytic cups in a retinal pigment epithelial-derived cell line (RPE1). Protrudin-dependent phagocytic cup formation required SYT7, which promotes fusion of LELys with the plasma membrane. RPE1 cells perform phagocytosis of dead cells (efferocytosis) that expose phosphatidylserine (PS) on their surface. Exogenous addition of apoptotic bodies increased the formation of phagocytic cups, which further increased when Protrudin was overexpressed. Overexpression of Protrudin also led to elevated uptake of silica beads coated with PS. Conversely, Protrudin depletion or abrogation of ER-endosome contact sites inhibited phagocytic cup formation resulting in reduced uptake of PS-coated beads. Thus, the Protrudin pathway delivers endosomes to facilitate formation of the phagocytic cup important for PS-dependent phagocytosis.
Keywords: Efferocytosis; Endosomes; FYCO1; Phagocytosis; Protrudin; RAB7; VAMP7.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Protrudin-mediated ER-endosome contact sites promote MT1-MMP exocytosis and cell invasion.J Cell Biol. 2020 Aug 3;219(8):e202003063. doi: 10.1083/jcb.202003063. J Cell Biol. 2020. PMID: 32479595 Free PMC article.
-
Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth.Nature. 2015 Apr 9;520(7546):234-8. doi: 10.1038/nature14359. Nature. 2015. PMID: 25855459
-
ER-endosome contact sites in endosome positioning and protrusion outgrowth.Biochem Soc Trans. 2016 Apr 15;44(2):441-6. doi: 10.1042/BST20150246. Biochem Soc Trans. 2016. PMID: 27068952 Review.
-
Lipid Transfer-Dependent Endosome Maturation Mediated by Protrudin and PDZD8 in Neurons.Front Cell Dev Biol. 2020 Dec 15;8:615600. doi: 10.3389/fcell.2020.615600. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33385000 Free PMC article. Review.
-
ER contact sites direct late endosome transport.Bioessays. 2015 Dec;37(12):1298-302. doi: 10.1002/bies.201500095. Epub 2015 Oct 6. Bioessays. 2015. PMID: 26440125 Review.
Cited by
-
The interactions of subcellular organelles in pulmonary fibrosis induced by carbon black nanoparticles: a comprehensive review.Arch Toxicol. 2024 Jun;98(6):1629-1643. doi: 10.1007/s00204-024-03719-0. Epub 2024 Mar 27. Arch Toxicol. 2024. PMID: 38536500 Review.
-
Lysosome-Mitochondrial Crosstalk in Cellular Stress and Disease.Antioxidants (Basel). 2025 Jan 22;14(2):125. doi: 10.3390/antiox14020125. Antioxidants (Basel). 2025. PMID: 40002312 Free PMC article. Review.
-
RILP Induces Cholesterol Accumulation in Lysosomes by Inhibiting Endoplasmic Reticulum-Endolysosome Interactions.Cells. 2024 Aug 6;13(16):1313. doi: 10.3390/cells13161313. Cells. 2024. PMID: 39195203 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

