Quantitative proteomics of cerebrospinal fluid from African Americans and Caucasians reveals shared and divergent changes in Alzheimer's disease
- PMID: 37468915
- PMCID: PMC10355042
- DOI: 10.1186/s13024-023-00638-z
Quantitative proteomics of cerebrospinal fluid from African Americans and Caucasians reveals shared and divergent changes in Alzheimer's disease
Abstract
Background: Despite being twice as likely to get Alzheimer's disease (AD), African Americans have been grossly underrepresented in AD research. While emerging evidence indicates that African Americans with AD have lower cerebrospinal fluid (CSF) levels of Tau compared to Caucasians, other differences in AD CSF biomarkers have not been fully elucidated. Here, we performed unbiased proteomic profiling of CSF from African Americans and Caucasians with and without AD to identify both common and divergent AD CSF biomarkers.
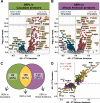
Methods: Multiplex tandem mass tag-based mass spectrometry (TMT-MS) quantified 1,840 proteins from 105 control and 98 AD patients of which 100 identified as Caucasian while 103 identified as African American. We used differential protein expression and co-expression approaches to assess how changes in the CSF proteome are related to race and AD. Co-expression network analysis organized the CSF proteome into 14 modules associated with brain cell-types and biological pathways. A targeted mass spectrometry method, selected reaction monitoring (SRM), with heavy labeled internal standards was used to measure a panel of CSF module proteins across a subset of African Americans and Caucasians with or without AD. A receiver operating characteristic (ROC) curve analysis assessed the performance of each protein biomarker in differentiating controls and AD by race.
Results: Consistent with previous findings, the increase of Tau levels in AD was greater in Caucasians than in African Americans by both immunoassay and TMT-MS measurements. CSF modules which included 14-3-3 proteins (YWHAZ and YWHAG) demonstrated equivalent disease-related elevations in both African Americans and Caucasians with AD, whereas other modules demonstrated more profound disease changes within race. Modules enriched with proteins involved with glycolysis and neuronal/cytoskeletal proteins, including Tau, were more increased in Caucasians than in African Americans with AD. In contrast, a module enriched with synaptic proteins including VGF, SCG2, and NPTX2 was significantly lower in African Americans than Caucasians with AD. Following SRM and ROC analysis, VGF, SCG2, and NPTX2 were significantly better at classifying African Americans than Caucasians with AD.
Conclusions: Our findings provide insight into additional protein biomarkers and pathways reflecting underlying brain pathology that are shared or differ by race.
Keywords: Amyloid; Biomarkers; CSF; Proteomics; Race; Tau.
© 2023. The Author(s).
Conflict of interest statement
A.I.L, N.T.S. and D.M.D. are co-founders of Emtherapro Inc.
Figures





References
-
- Lines L, Sherif N, Wiener J. Racial and ethnic disparities among individuals with Alzheimer’s disease in the United States: A literature review RTI Press. 2014 doi: 10.3768/rtipress.2014.RR.0024.1412. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

