Single-cell transcriptome sequencing-based analysis: probing the mechanisms of glycoprotein NMB regulation of epithelial cells involved in silicosis
- PMID: 37468937
- PMCID: PMC10354944
- DOI: 10.1186/s12989-023-00543-9
Single-cell transcriptome sequencing-based analysis: probing the mechanisms of glycoprotein NMB regulation of epithelial cells involved in silicosis
Abstract
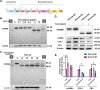
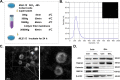
Chronic exposure to silica can lead to silicosis, one of the most serious occupational lung diseases worldwide, for which there is a lack of effective therapeutic drugs and tools. Epithelial mesenchymal transition plays an important role in several diseases; however, data on the specific mechanisms in silicosis models are scarce. We elucidated the pathogenesis of pulmonary fibrosis via single-cell transcriptome sequencing and constructed an experimental silicosis mouse model to explore the specific molecular mechanisms affecting epithelial mesenchymal transition at the single-cell level. Notably, as silicosis progressed, glycoprotein non-metastatic melanoma protein B (GPNMB) exerted a sustained amplification effect on alveolar type II epithelial cells, inducing epithelial-to-mesenchymal transition by accelerating cell proliferation and migration and increasing mesenchymal markers, ultimately leading to persistent pulmonary pathological changes. GPNMB participates in the epithelial-mesenchymal transition in distant lung epithelial cells by releasing extracellular vesicles to accelerate silicosis. These vesicles are involved in abnormal changes in the composition of the extracellular matrix and collagen structure. Our results suggest that GPNMB is a potential target for fibrosis prevention.
Keywords: Epithelial mesenchymal transformation; Extracellular matrix; Extracellular vesicles; Pulmonary fibrosis; Silica.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
LncRNA UCA1 regulates silicosis-related lung epithelial cell-to-mesenchymal transition through competitive adsorption of miR-204-5p.Toxicol Appl Pharmacol. 2022 Apr 15;441:115977. doi: 10.1016/j.taap.2022.115977. Epub 2022 Mar 11. Toxicol Appl Pharmacol. 2022. PMID: 35288145
-
MiR-26a-5p from HucMSC-derived extracellular vesicles inhibits epithelial mesenchymal transition by targeting Adam17 in silica-induced lung fibrosis.Ecotoxicol Environ Saf. 2023 Jun 1;257:114950. doi: 10.1016/j.ecoenv.2023.114950. Epub 2023 Apr 24. Ecotoxicol Environ Saf. 2023. PMID: 37099959
-
miR-138 inhibits epithelial-mesenchymal transition in silica-induced pulmonary fibrosis by regulating ZEB2.Toxicology. 2021 Sep;461:152925. doi: 10.1016/j.tox.2021.152925. Epub 2021 Sep 2. Toxicology. 2021. PMID: 34481903
-
[Progress in study on intervention of silica-induced pulmonary fibrosis with the exosomes released from mesenchymal stem cells].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020 Apr 20;38(4):309-313. doi: 10.3760/cma.j.cn121094-20190709-00275. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020. PMID: 32447901 Review. Chinese.
-
Autophagy, an important therapeutic target for pulmonary fibrosis diseases.Clin Chim Acta. 2020 Mar;502:139-147. doi: 10.1016/j.cca.2019.12.016. Epub 2019 Dec 23. Clin Chim Acta. 2020. PMID: 31877297 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

