Proton therapy and oral mucositis in oral & oropharyngeal cancers: outcomes, dosimetric and NTCP benefit
- PMID: 37468950
- PMCID: PMC10357709
- DOI: 10.1186/s13014-023-02317-1
Proton therapy and oral mucositis in oral & oropharyngeal cancers: outcomes, dosimetric and NTCP benefit
Abstract
Introduction: Radiation-induced oral mucositis (RIOM), is a common, debilitating, acute side effect of radiotherapy for oral cavity (OC) and oropharyngeal (OPx) cancers; technical innovations for reducing it are seldom discussed. Intensity-modulated-proton-therapy (IMPT) has been reported extensively for treating OPx cancers, and less frequently for OC cancers. We aim to quantify the reduction in the likelihood of RIOM in treating these 2 subsites with IMPT compared to Helical Tomotherapy.
Material and methods: We report acute toxicities and early outcomes of 22 consecutive patients with OC and OPx cancers treated with IMPT, and compare the dosimetry and normal tissue complication probability (NTCP) of ≥ grade 3 mucositis for IMPT and HT.
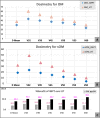
Results: Twenty two patients, 77% males, 41% elderly and 73% OC subsite, were reviewed. With comparable target coverage, IMPT significantly reduced the mean dose and D32, D39, D45, and D50, for both the oral mucosa (OM) and spared oral mucosa (sOM). With IMPT, there was a 7% absolute and 16.5% relative reduction in NTCP for grade 3 mucositis for OM, compared to HT. IMPT further reduced NTCP for sOM, and the benefit was maintained in OC, OPx subsites and elderly subgroup. Acute toxicities, grade III dermatitis and mucositis, were noted in 50% and 45.5% patients, respectively, while 22.7% patients had grade 3 dysphagia. Compared with published data, the hospital admission rate, median weight loss, feeding tube insertion, unplanned treatment gaps were lower with IMPT. At a median follow-up of 15 months, 81.8% were alive; 72.7%, alive without disease and 9%, alive with disease.
Conclusion: The dosimetric benefit of IMPT translates into NTCP reduction for grade 3 mucositis compared to Helical Tomotherapy for OPx and OC cancers and encourages the use of IMPT in their management.
Keywords: Mucositis; NTCP; Oral cavity; Oropharyngeal; Proton.
© 2023. The Author(s).
Conflict of interest statement
The authors declares that they have no competing interests.
Figures

Similar articles
-
NTCP reduction for advanced head and neck cancer patients using proton therapy for complete or sequential boost treatment versus photon therapy.Acta Oncol. 2015;54(9):1658-64. doi: 10.3109/0284186X.2015.1071920. Epub 2015 Sep 4. Acta Oncol. 2015. PMID: 26340301
-
Spot scanning proton arc therapy reduces toxicity in oropharyngeal cancer patients.Med Phys. 2023 Mar;50(3):1305-1317. doi: 10.1002/mp.16098. Epub 2023 Jan 17. Med Phys. 2023. PMID: 36373893
-
Dosimetric advantages of intensity-modulated proton therapy for oropharyngeal cancer compared with intensity-modulated radiation: A case-matched control analysis.Med Dosim. 2016 Autumn;41(3):189-94. doi: 10.1016/j.meddos.2016.01.002. Epub 2016 May 4. Med Dosim. 2016. PMID: 27158021
-
Intensity modulated proton therapy (IMPT) - The future of IMRT for head and neck cancer.Oral Oncol. 2019 Jan;88:66-74. doi: 10.1016/j.oraloncology.2018.11.015. Epub 2018 Nov 21. Oral Oncol. 2019. PMID: 30616799 Free PMC article. Review.
-
Proton Therapy for HPV-Associated Oropharyngeal Cancers of the Head and Neck: a De-Intensification Strategy.Curr Treat Options Oncol. 2021 Jun 4;22(6):54. doi: 10.1007/s11864-021-00847-y. Curr Treat Options Oncol. 2021. PMID: 34086150 Free PMC article. Review.
Cited by
-
Systematic review of the tools and outcomes for the assessment of acute radiation dermatitis severity.Clin Transl Radiat Oncol. 2025 May 9;53:100977. doi: 10.1016/j.ctro.2025.100977. eCollection 2025 Jul. Clin Transl Radiat Oncol. 2025. PMID: 40502854 Free PMC article. Review.
-
Dosimetric benefits of customised mouth-bite for head neck cancer patients undergoing modern proton therapy - An audit.Tech Innov Patient Support Radiat Oncol. 2024 Jul 31;31:100265. doi: 10.1016/j.tipsro.2024.100265. eCollection 2024 Sep. Tech Innov Patient Support Radiat Oncol. 2024. PMID: 39224831 Free PMC article.
References
-
- Bowen J, Al-Dasooqi N, Bossi P, Wardill H, Van Sebille Y, Al-Azri A, Bateman E, Correa ME, Raber-Durlacher J, Kandwal A, Mayo B, Nair RG, Stringer A, Ten Bohmer K, Thorpe D, Lalla RV, Sonis S, Cheng K, Elad S. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). The pathogenesis of mucositis: updated perspectives and emerging targets. Support Care Cancer. 2019;27(10):4023–4033. doi: 10.1007/s00520-019-04893-z. - DOI - PubMed
-
- Bar AdV, Weinstein G, Dutta PR, Dosoretz A, Chalian A, Both S, Quon H. Gabapentin for the treatment of pain syndrome related to radiation-induced mucositis in patients with head and neck cancer treated with concurrent chemoradiotherapy. Cancer. 2010;116(17):4206–4213. doi: 10.1002/cncr.25274. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

