Does each Component of Reactive Oxygen Species have a Dual Role in the Tumor Microenvironment?
- PMID: 37469162
- PMCID: PMC11340293
- DOI: 10.2174/0929867331666230719142202
Does each Component of Reactive Oxygen Species have a Dual Role in the Tumor Microenvironment?
Abstract
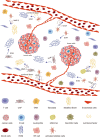
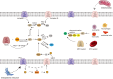
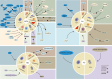
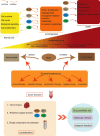
Reactive oxygen species (ROS) are a class of highly reactive oxidizing molecules, including superoxide anion (O2 •-) and hydrogen peroxide (H2O2), among others. Moderate levels of ROS play a crucial role in regulating cellular signaling and maintaining cellular functions. However, abnormal ROS levels or persistent oxidative stress can lead to changes in the tumor microenvironment (TME) that favor cancer development. This review provides an overview of ROS generation, structure, and properties, as well as their effects on various components of the TME. Contrary to previous studies, our findings reveal a dual effect of ROS on different components of the TME, whereby ROS can either enhance or inhibit certain factors, ultimately leading to the promotion or suppression of the TME. For example, H2O2 has dual effects on immune cells and non-- cellular components within the TME, while O2 •- has dual effects on T cells and fibroblasts. Furthermore, each component demonstrates distinct mechanisms of action and ranges of influence. In the final section of the article, we summarize the current clinical applications of ROS in cancer treatment and identify certain limitations associated with existing therapeutic approaches. Therefore, this review aims to provide a comprehensive understanding of ROS, highlighting their dual effects on different components of the TME, and exploring the potential clinical applications that may pave the way for future treatment and prevention strategies.
Keywords: ROS; metabolism; microenvironment; molecule; therapy.; tumor.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures
References
-
- Weinberg F., Hamanaka R., Wheaton W.W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G.M., Budinger G.R.S., Chandel N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA. 2010;107(19):8788–8793. doi: 10.1073/pnas.1003428107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

