Pharmacokinetics and tolerability of multiple-day oral dosing of mycophenolate mofetil in healthy horses
- PMID: 37469186
- PMCID: PMC10472989
- DOI: 10.1111/jvim.16797
Pharmacokinetics and tolerability of multiple-day oral dosing of mycophenolate mofetil in healthy horses
Abstract
Background: Additional efficacious immunomodulatory treatment is needed for the management of immune-mediated disease in horses. Mycophenolate mofetil (MMF) is an immunosuppressive drug that warrants assessment as a viable therapeutic agent for horses.
Hypothesis/objectives: To evaluate the pharmacokinetics (PK) of multiple-day oral dosing of MMF in healthy horses and to determine the tolerability of this dosing regimen.
Animals: Six healthy Standardbred mares.
Methods: Horses received MMF 10 mg/kg PO q12h for 7 days in the fed state. Serial sampling was performed over 12 hours on Days 1 and 7 with trough samples collected every 24 hours, immediately before morning drug administration. Noncompartmental PK analyses were performed to determine primary PK parameters, followed by calculation of geometric means and coefficients of variation. A CBC, serum biochemical profile, physical examination, and fecal scoring were used to assess dose tolerability.
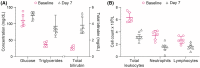
Results: Seven days of treatment resulted in a mycophenolic acid (MPA) area under the curve (AUC0-12 ) of 12 594 h × ng/mL (8567-19 488 h × ng/mL) and terminal half-life (T1/2 ) of 11.3 hours (7.5-15.9 hours), yielding minor metabolite accumulation in all horses treated. Salmonellosis was detected in the feces of 2 horses by Day 7, and all horses developed myelosuppression, hyperbilirubinemia, hyporexia, decreased gastrointestinal motility, and decreased fecal output by the seventh day of treatment.
Conclusion and clinical importance: Administration of MMF at 10 mg/kg PO q12h resulted in hematologic and clinical toxicity within 1 week of treatment. A decreased MMF dose, frequency, or both is needed to avoid colic. Drug monitoring should include frequent hemograms, serum biochemical profiles, and strict biosecurity protocols.
Keywords: dermatology; equine; immune-mediated; lymphopenia; neutropenia.
© 2023 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Gwendolen Lorch is a scientific board member of Vidium Animal Health. No other authors declare a conflict of interest.
Figures

References
-
- Leclere M. Corticosteroids and immune suppressive therapies in horses. Vet Clin North Am Equine Pract. 2017;33:17‐27. - PubMed
-
- Abd Rahman AN, Tett SE, Staatz CE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in patients with autoimmune disease. Clin Pharmacokinet. 2013;52:303‐331. - PubMed
-
- Dewey CW, Cerda‐Gonzalez S, Fletcher DJ, et al. Mycophenolate mofetil treatment in dogs with serologically diagnosed acquired myasthenia gravis: 27 cases (1999‐2008). J Am Vet Med Assoc. 2010;236:664‐668. - PubMed
-
- Orvis AK, Wesson SK, Breza TS, et al. Mycophenolate mofetil in dermatology. J Am Acad Dermatol. 2009;60:183‐199; quiz 200–182. - PubMed
-
- Wang A, Smith JR, Creevy KE. Treatment of canine idiopathic immune‐mediated haemolytic anaemia with mycophenolate mofetil and glucocorticoids: 30 cases (2007 to 2011). J Small Anim Pract. 2013;54:399‐404. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

