Skeletal muscle-specific DJ-1 ablation-induced atrogenes expression and mitochondrial dysfunction contributing to muscular atrophy
- PMID: 37469245
- PMCID: PMC10570112
- DOI: 10.1002/jcsm.13290
Skeletal muscle-specific DJ-1 ablation-induced atrogenes expression and mitochondrial dysfunction contributing to muscular atrophy
Abstract
Background: DJ-1 is a causative gene for Parkinson's disease. DJ-1-deficient mice develop gait-associated progressive behavioural abnormalities and hypoactive forearm grip strength. However, underlying activity mechanisms are not fully explored.
Methods: Western blotting and quantitative real-time polymerase chain reaction approaches were adopted to analyse DJ-1 expression in skeletal muscle from aged humans or mice and compared with young subjects. Skeletal muscle-specific-DJ-1 knockout (MDKO) mice were generated, followed by an assessment of the physical activity phenotypes (grip strength, maximal load capacity, and hanging, rotarod, and exercise capacity tests) of the MDKO and control mice on the chow diet. Muscular atrophy phenotypes (cross-sectional area and fibre types) were determined by imaging and quantitative real-time polymerase chain reaction. Mitochondrial function and skeletal muscle morphology were evaluated by oxygen consumption rate and electron microscopy, respectively. Tail suspension was applied to address disuse atrophy. RNA-seq analysis was performed to indicate molecular changes in muscles with DJ-1 ablation. Dual-luciferase reporter assays were employed to identify the promoter region of Trim63 and Fbxo32 genes, which were indirectly regulated by DJ-1 via the FoxO1 pathway. Cytoplasmic and nuclear fractions of DJ-1-deleted muscle cells were analysed by western blotting. Compound 23 was administered into the gastrocnemius muscle to mimic the of DJ-1 deletion effects.
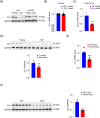
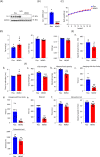
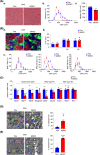
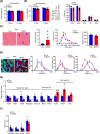
Results: DJ-1 expression decreased in atrophied muscles of aged human (young men, n = 2; old with aged men, n = 2; young women, n = 2; old with aged women, n = 2) and immobilization mice (n = 6, P < 0.01). MDKO mice exhibited no body weight difference compared with control mice on the chow diet (Flox, n = 8; MDKO, n = 9). DJ-1-deficient muscles were slightly dystrophic (Flox, n = 7; MDKO, n = 8; P < 0.05), with impaired physical activities and oxidative capacity (n = 8, P < 0.01). In disuse-atrophic conditions, MDKO mice showed smaller cross-sectional area (n = 5, P < 0.01) and more central nuclei than control mice (Flox, n = 7; MDKO, n = 6; P < 0.05), without alteration in muscle fibre types (Flox, n = 6; MDKO, n = 7). Biochemical analysis indicated that reduced mitochondrial function and upregulated of atrogenes induced these changes. Furthermore, RNA-seq analysis revealed enhanced activity of the FoxO1 signalling pathway in DJ-1-ablated muscles, which was responsible for the induction of atrogenes. Finally, compound 23 (an inhibitor of DJ-1) could mimic the effects of DJ-1 ablation in vivo.
Conclusions: Our results illuminate the crucial of skeletal muscle DJ-1 in the regulation of catabolic signals from mechanical stimulation, providing a therapeutic target for muscle wasting diseases.
Keywords: Atrogenes; Atrophy; DJ-1; Skeletal muscle.
© 2023 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin‐proteasome pathway. N Engl J Med 1996;335:1897–1905. - PubMed
-
- Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone‐treated skeletal muscle. Cell Metab 2007;6:376–385. - PubMed
-
- Attaix D, Baracos VE. MAFbx/Atrogin‐1 expression is a poor index of muscle proteolysis. Curr Opin Clin Nutr Metab Care 2010;13:223–224. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

