Camrelizumab as a novel third or post-third-line treatment strategy in small cell lung cancer: a retrospective study of 12 patients
- PMID: 37469402
- PMCID: PMC10352824
- DOI: 10.3389/fonc.2023.1180735
Camrelizumab as a novel third or post-third-line treatment strategy in small cell lung cancer: a retrospective study of 12 patients
Abstract
Background: Small cell lung cancer (SCLC) constitutes 15% of all lung cancer cases, with a comparatively low survival rate. The advent of immune checkpoint inhibitors (ICIs) has provided new alternatives for treating SCLC. However, the effectiveness of camrelizumab in the treatment of SCLC remains unclear. This retrospective case series was designed to investigate the efficacy and safety of camrelizumab in SCLC patients.
Methods: The study enrolled SCLC patients recorded as having received more than one cycle of camrelizumab in the electronic medical record system. Data related to clinical and survival times were collected and statistically analyzed.
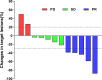
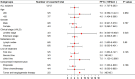
Results: From August 2019 to December 2021, the study enrolled 12 SCLC patients. The objective response rate was 41.7% (95% confidence interval [CI]: 15.2%-72.3%). The disease control rate was 83.3% (95% CI: 51.6%-97.9%). The median progression-free survival (PFS) for all patients was 4.0 months. Notably, the median PFS of patients in third- or post-third-line subgroups was 7 months (95% CI: 1.12-12.88 months). The median overall survival (OS) for all eligible patients was 10.0 months (95% CI: 7.35-12.65 months), with a 1-year survival rate of 25%. Notably, the OS of patients treated with third- or post-third-line therapy was 5-34 months, with a 1-year survival rate of 75%. The two most prevalent non-hematological adverse events associated with the immune response were pneumonitis (44.4%) and reactive cutaneous capillary endothelial proliferation (44.4%). One patient experienced an exacerbation of preexisting diabetes and reached grade 3 hyperglycemia. There were no grade 4/5 immune-related adverse events.
Conclusion: This case series highlights the potential benefits and safety concerns of camrelizumab in SCLC patients. These findings suggest a possible strategy for third- and post-third-line treatments of SCLC. However, the conclusion is limited due to the study's retrospective nature and small sample size. Therefore, large-scale randomized controlled studies are needed to determine its efficacy.
Keywords: PD1 inhibitor; SCLC; camrelizumab; case series; chemotherapy.
Copyright © 2023 Tian, Sui, Wang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources