Unresectable stage III non-small cell lung cancer: could durvalumab be safe and effective in real-life clinical scenarios? Results of a single-center experience
- PMID: 37469420
- PMCID: PMC10352832
- DOI: 10.3389/fonc.2023.1208204
Unresectable stage III non-small cell lung cancer: could durvalumab be safe and effective in real-life clinical scenarios? Results of a single-center experience
Abstract
Introduction: The standard of care for patients with unresectable stage III non-small cell lung cancer (NSCLC) is chemoradiotherapy (CRT) followed by consolidation durvalumab as shown in the PACIFIC trial. The purpose of this study is to evaluate clinical outcomes and toxicities regarding the use of durvalumab in a real clinical scenario.
Methods: A single-center retrospective study was conducted on patients with a diagnosis of unresectable stage III NSCLC who underwent radical CRT followed or not by durvalumab. Tumor response after CRT, pattern of relapse, overall survival (OS) and progression-free survival (PFS), and toxicity profile were investigated.
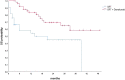
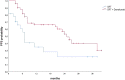
Results: Eighty-five patients met the inclusion criteria. The median age was 67 years (range 45-82 years). Fifty-two patients (61.2%) started sequential therapy with durvalumab. The main reason for excluding patients from the durvalumab treatment was the expression of PD-L1 < 1%. Only two patients presented a grade 4 or 5 pneumonitis. A median follow-up (FU) of 20 months has been reached. Forty-five patients (52.9%) had disease progression, and 21 (24.7%) had a distant progression. The addition of maintenance immunotherapy confirmed a clinical benefit in terms of OS and PFS. Two-year OS and PFS were respectively 69.4% and 54.4% in the durvalumab group and 47.9% and 24.2% in the no-durvalumab group (p = 0.015, p = 0.007).
Conclusion: In this real-world study, patients treated with CRT plus durvalumab showed clinical outcomes and toxicities similar to the PACIFIC results. Maintenance immunotherapy after CRT has been shown to be safe and has increased the survival of patients in clinical practice.
Keywords: chemo-radiotherapy (CRT); durvalumab; non-small cell lung cancer (NSCLC); real-world data (RWD); stage III.
Copyright © 2023 Borghetti, Volpi, Facheris, Cossali, Mataj, La Mattina, Singh, Imbrescia, Bonù, Tomasini, Vitali, Greco, Bezzi, Melotti, Benvenuti, Borghesi, Grisanti and Buglione di Monale e Bastia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Real-World Insights into the Impact of Durvalumab on Stage III Unresectable Non-Small Cell Lung Cancer-A Narrative Review.Cancers (Basel). 2025 Mar 3;17(5):874. doi: 10.3390/cancers17050874. Cancers (Basel). 2025. PMID: 40075721 Free PMC article. Review.
-
A Real-World, Multicenter, Observational Retrospective Study of Durvalumab After Concomitant or Sequential Chemoradiation for Unresectable Stage III Non-Small Cell Lung Cancer.Front Oncol. 2021 Sep 28;11:744956. doi: 10.3389/fonc.2021.744956. eCollection 2021. Front Oncol. 2021. PMID: 34650927 Free PMC article.
-
Novel imaging biomarkers predict outcomes in stage III unresectable non-small cell lung cancer treated with chemoradiation and durvalumab.J Immunother Cancer. 2022 Mar;10(3):e003778. doi: 10.1136/jitc-2021-003778. J Immunother Cancer. 2022. PMID: 35256515 Free PMC article.
-
Real-world outcomes with durvalumab after chemoradiotherapy in patients with unresectable stage III NSCLC: interim analysis of overall survival from PACIFIC-R.ESMO Open. 2024 Jun;9(6):103464. doi: 10.1016/j.esmoop.2024.103464. Epub 2024 Jun 3. ESMO Open. 2024. PMID: 38833971 Free PMC article.
-
Real-World Safety and Efficacy of Consolidation Durvalumab After Chemoradiation Therapy for Stage III Non-small Cell Lung Cancer: A Systematic Review and Meta-analysis.Int J Radiat Oncol Biol Phys. 2022 Apr 1;112(5):1154-1164. doi: 10.1016/j.ijrobp.2021.12.150. Epub 2021 Dec 26. Int J Radiat Oncol Biol Phys. 2022. PMID: 34963558
Cited by
-
Evaluation of the efficacy and safety of durvalumab combined with chemotherapy, radiotherapy, or other agents in advanced non-small cell lung cancer: a meta-analysis.Int J Clin Exp Pathol. 2025 Jun 15;18(6):203-221. doi: 10.62347/BNVX1803. eCollection 2025. Int J Clin Exp Pathol. 2025. PMID: 40642395 Free PMC article. Review.
-
Real-World Treatment Patterns and Timeliness of Clinical Care Pathway for Non-Small Cell Lung Cancer Patients in Austria: The PRATER Retrospective Study.Cancers (Basel). 2024 Jul 19;16(14):2586. doi: 10.3390/cancers16142586. Cancers (Basel). 2024. PMID: 39061224 Free PMC article.
-
Immunotherapy Improves Clinical Outcome in Kirsten Rat Sarcoma Virus-Mutated Patients with Unresectable Non-Small Cell Lung Cancer Stage III: A Subcohort Analysis of the Austrian Radio-Oncological Lung Cancer Study Association Registry (ALLSTAR).J Clin Med. 2025 Feb 1;14(3):945. doi: 10.3390/jcm14030945. J Clin Med. 2025. PMID: 39941616 Free PMC article.
-
Real-World Insights into the Impact of Durvalumab on Stage III Unresectable Non-Small Cell Lung Cancer-A Narrative Review.Cancers (Basel). 2025 Mar 3;17(5):874. doi: 10.3390/cancers17050874. Cancers (Basel). 2025. PMID: 40075721 Free PMC article. Review.
-
Durvalumab Prolongs Overall Survival, Whereas Radiation Dose Escalation > 66 Gy Might Improve Long-Term Local Control in Unresectable NSCLC Stage III: Updated Analysis of the Austrian Radio-Oncological Lung Cancer Study Association Registry (ALLSTAR).Cancers (Basel). 2025 Apr 25;17(9):1443. doi: 10.3390/cancers17091443. Cancers (Basel). 2025. PMID: 40361370 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous