Analysis benefits of a second Allo-HSCT after CAR-T cell therapy in patients with relapsed/refractory B-cell acute lymphoblastic leukemia who relapsed after transplant
- PMID: 37469510
- PMCID: PMC10352576
- DOI: 10.3389/fimmu.2023.1191382
Analysis benefits of a second Allo-HSCT after CAR-T cell therapy in patients with relapsed/refractory B-cell acute lymphoblastic leukemia who relapsed after transplant
Abstract
Background: Chimeric antigen receptor (CAR) T-cell therapy has demonstrated high initial complete remission (CR) rates in B-cell acute lymphoblastic leukemia (B-ALL) patients, including those who relapsed after transplant. However, the duration of remission requires improvements. Whether bridging to a second allogeneic hematopoietic stem cell transplant (allo-HSCT) after CAR-T therapy can improve long-term survival remains controversial. We retrospectively analyzed long-term follow-up data of B-ALL patients who relapsed post-transplant and received CAR-T therapy followed by consolidation second allo-HSCT to investigate whether such a treatment sequence could improve long-term survival.
Methods: A single-center, retrospective study was performed between October 2017 and March 2022, involving 95 patients who received a consolidation second transplant after achieving CR from CAR-T therapy.
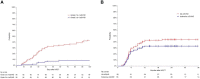
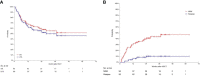
Results: The median age of patients was 22.8 years (range: 3.3-52.8) at the second transplant. After the first transplant, 71 patients (74.7%) experienced bone marrow relapse, 16 patients (16.8%) had extramedullary relapse, 5 patients (5.3%) had both bone marrow and extramedullary relapse and 3/95 patients (3.2%) had positive minimal residual disease (MRD) only. Patients received autologous (n=57, 60.0%) or allogeneic (n=28, 29.5%) CAR-T cells, while 10 patients (10.5%) were unknown. All patients achieved CR after CAR-T therapy. Before second HSCT, 86 patients (90.5%) were MRD-negative, and 9 (9.5%) were MRD-positive. All second transplant donors were different from the first transplant donors. The median follow-up time was 623 days (range: 33-1901) after the second HSCT. The 3-year overall survival (OS) and leukemia-free survival (LFS) were 55.3% (95%CI, 44.3-66.1%) and 49.8% (95%CI, 38.7-60.9%), respectively. The 3-year relapse incidence (RI) and non-relapse mortality (NRM) were 10.5% (95%CI, 5.6-19.6%) and 43.6% (95%CI, 33.9-56.2%), respectively. In multivariate analysis, the interval from CAR-T to second HSCT ≤90 days was associated with superior LFS(HR, 4.10, 95%CI,1.64-10.24; p=0.003) and OS(HR, 2.67, 95%CI, 1.24-5.74, p=0.012), as well as reduced NRM (HR, 2.45, 95%CI, 1.14-5.24, p=0.021).
Conclusions: Our study indicated that CAR-T therapy followed by consolidation second transplant could significantly improve long-term survival in B-ALL patients who relapsed post-transplant. The second transplant should be considered in suitable patients and is recommended to be performed within 90 days after CAR-T treatment.
Keywords: B-ALL; CAR-T; LFS; NRM; relapse; second transplant.
Copyright © 2023 Cao, Zhang, Zhao, Xiong, Zhou, Lu, Sun, Wei, Liu, Zhang, Yang and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Integrating CAR T-Cell Therapy and Transplantation: Comparisons of Safety and Long-Term Efficacy of Allogeneic Hematopoietic Stem Cell Transplantation After CAR T-Cell or Chemotherapy-Based Complete Remission in B-Cell Acute Lymphoblastic Leukemia.Front Immunol. 2021 May 7;12:605766. doi: 10.3389/fimmu.2021.605766. eCollection 2021. Front Immunol. 2021. PMID: 34025637 Free PMC article.
-
Humanized Anti-CD19 CAR-T Cell Therapy and Sequential Allogeneic Hematopoietic Stem Cell Transplantation Achieved Long-Term Survival in Refractory and Relapsed B Lymphocytic Leukemia: A Retrospective Study of CAR-T Cell Therapy.Front Immunol. 2021 Oct 29;12:755549. doi: 10.3389/fimmu.2021.755549. eCollection 2021. Front Immunol. 2021. PMID: 34777367 Free PMC article.
-
Comparable outcomes in patients with B-cell acute lymphoblastic leukemia receiving haploidentical hematopoietic stem cell transplantation: Pretransplant minimal residual disease-negative complete remission following chimeric antigen receptor T-cell therapy versus chemotherapy.Front Immunol. 2022 Aug 30;13:934442. doi: 10.3389/fimmu.2022.934442. eCollection 2022. Front Immunol. 2022. PMID: 36110859 Free PMC article.
-
How to Combine the Two Landmark Treatment Methods-Allogeneic Hematopoietic Stem Cell Transplantation and Chimeric Antigen Receptor T Cell Therapy Together to Cure High-Risk B Cell Acute Lymphoblastic Leukemia?Front Immunol. 2020 Dec 15;11:611710. doi: 10.3389/fimmu.2020.611710. eCollection 2020. Front Immunol. 2020. PMID: 33384696 Free PMC article. Review.
-
Efficacy and safety of CD19 CAR-T cell therapy for acute lymphoblastic leukemia patients relapsed after allogeneic hematopoietic stem cell transplantation.Int J Hematol. 2022 Sep;116(3):315-329. doi: 10.1007/s12185-022-03398-6. Epub 2022 Jun 23. Int J Hematol. 2022. PMID: 35737192 Review.
Cited by
-
Comprehensive analysis of the efficacy and safety of CAR T-cell therapy in patients with relapsed or refractory B-cell acute lymphoblastic leukaemia: a systematic review and meta-analysis.Ann Med. 2024 Dec;56(1):2349796. doi: 10.1080/07853890.2024.2349796. Epub 2024 May 13. Ann Med. 2024. PMID: 38738799 Free PMC article.
-
CAR-T Cell Therapy for Acute Myeloid Leukemia: Where Do We Stand Now?Curr Oncol. 2025 May 30;32(6):322. doi: 10.3390/curroncol32060322. Curr Oncol. 2025. PMID: 40558265 Free PMC article. Review.
-
Granulocyte Colony Stimulating Factor-Mobilized Peripheral Blood Mononuclear Cells: An Alternative Cellular Source for Chimeric Antigen Receptor Therapy.Int J Mol Sci. 2024 May 25;25(11):5769. doi: 10.3390/ijms25115769. Int J Mol Sci. 2024. PMID: 38891957 Free PMC article. Review.
-
CAR-T Cell Therapy in B-Cell Acute Lymphoblastic Leukemia.Mediterr J Hematol Infect Dis. 2024 Jan 1;16(1):e2024010. doi: 10.4084/MJHID.2024.010. eCollection 2024. Mediterr J Hematol Infect Dis. 2024. PMID: 38223477 Free PMC article. Review.
References
-
- Nagler A, Labopin M, Houhou M, Aljurf M, Mousavi A, Hamladji RM, et al. . Outcome of haploidentical versus matched sibling donors in hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia:a study from the acute leukemia working party of the European society for blood and marrow transplantation. J Hematol Oncol (2021) 14:53. doi: 10.1186/s13045-021-01065-7 - DOI - PMC - PubMed
-
- Pavlu J, Labopin M, Niittyvuopio R, Socie G, Yakoub-Agha I, Wu D, et al. . Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia:a retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. J Hematol Oncol (2019) 12:108. doi: 10.1186/s13045-019-0790-x - DOI - PMC - PubMed
-
- Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M, et al. . Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. an analysis on behalf of the acute leukemia working party of EBMT. Leukemia (2012) 26:1211–17. doi: 10.1038/leu.2011.351 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

