CRISPR/Cas9 as a therapeutic tool for triple negative breast cancer: from bench to clinics
- PMID: 37469704
- PMCID: PMC10352522
- DOI: 10.3389/fmolb.2023.1214489
CRISPR/Cas9 as a therapeutic tool for triple negative breast cancer: from bench to clinics
Abstract
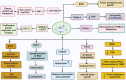
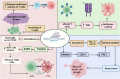
Clustered regularly interspaced short palindromic repeats (CRISPR) is a third-generation genome editing method that has revolutionized the world with its high throughput results. It has been used in the treatment of various biological diseases and infections. Various bacteria and other prokaryotes such as archaea also have CRISPR/Cas9 systems to guard themselves against bacteriophage. Reportedly, CRISPR/Cas9-based strategy may inhibit the growth and development of triple-negative breast cancer (TNBC) via targeting the potentially altered resistance genes, transcription, and epigenetic regulation. These therapeutic activities could help with the complex issues such as drug resistance which is observed even in TNBC. Currently, various methods have been utilized for the delivery of CRISPR/Cas9 into the targeted cell such as physical (microinjection, electroporation, and hydrodynamic mode), viral (adeno-associated virus and lentivirus), and non-viral (liposomes and lipid nano-particles). Although different models have been developed to investigate the molecular causes of TNBC, but the lack of sensitive and targeted delivery methods for in-vivo genome editing tools limits their clinical application. Therefore, based on the available evidences, this review comprehensively highlighted the advancement, challenges limitations, and prospects of CRISPR/Cas9 for the treatment of TNBC. We also underscored how integrating artificial intelligence and machine learning could improve CRISPR/Cas9 strategies in TNBC therapy.
Keywords: CRISPR/Cas9; drug resistance and artificial intelligence; gene editing; immunotherapy; triple negative breast cancer.
Copyright © 2023 Tiwari, Ko, Dubey, Chouhan, Tsai, Singh, Chaubey, Dayal, Chiang and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

