Improving performance robustness of subject-based brain segmentation software
- PMID: 37469714
- PMCID: PMC10295817
- DOI: 10.47936/encephalitis.2022.00108
Improving performance robustness of subject-based brain segmentation software
Abstract
Purpose: Artificial intelligence (AI)-based image analysis tools to quantify the brain have become commercialized. However, insufficient data for learning and scanner specificity is a limitation for achieving high quality. In the present study, the performance of personalized brain segmentation software when applied to multicenter data using an AI model trained on data from a single institution was improved.
Methods: Preindicators of brain white matter (WM) information from the training dataset were utilized for preprocessing. During learning, data of cognitively normal (CN) individuals from a single center were utilized, and data of CN individuals and Alzheimer disease (AD) patients enrolled in multiple centers were considered the test set.
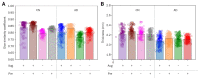
Results: The preprocessing based on the preindicator (dice similarity coefficient [DSC], 0.8567) resulted in a better performance than without (DSC, 0.7921). The standard deviation (SD) of the WM region intensity (DSC, 0.8303) had a more substantial influence on the performance than the average intensity (DSC, 0.6591). When the SD of the test data WM intensity was smaller than the learning data, the performance improved (0.03 increase in lower SD, 0.05 decrease in higher SD). Furthermore, preindicator-based pretreatment increased the correlation of mean cortical thickness of the entire gray matter between Atroscan and FreeSurfer, and data augmentation without preprocessing did not.Both preindicator processing and data augmentation improved the correlation coefficient from 0.7584 to 0.8165.
Conclusion: Data augmentation and preindicator-based preprocessing of training data can improve the performance of AI-based brain segmentation software, both increasing the generalizability and stability of brain segmentation software.
Keywords: Alzheimer disease; Artificial intelligence; Data augmentation; Segmentation.
Copyright © 2023 Korean Encephalitis and Neuroinflammation Society.
Conflict of interest statement
Conflicts of Interest Park JH, Kim D, Lee MJ, and Kang S are employees of the JLK. All were blinded to group allocation. Kang SJ, Yoon DH, Lee SK, and Park KI have nothing to disclose. Additional information for correspondence and requests for data should be addressed to Park KI.
Figures



References
-
- Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ, Utrecht Diabetic Encephalopathy Study Group Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci. 2010;299:126–130. - PubMed
-
- Khedher L, Ramírez J, Górriz JM, Brahim A, Segovia F, Alzheimer’s Disease Neuroimaging Initiative Early diagnosis of Alzheimer’s disease based on partial least squares, principal component analysis and support vector machine using segmented MRI images. Neurocomputing. 2015;151(Part 1):139–150.
LinkOut - more resources
Full Text Sources

