Tuberculosis-induced aplastic crisis and atypical lymphocyte expansion in advanced myelodysplastic syndrome: A case report and review of literature
- PMID: 37469724
- PMCID: PMC10353497
- DOI: 10.12998/wjcc.v11.i19.4713
Tuberculosis-induced aplastic crisis and atypical lymphocyte expansion in advanced myelodysplastic syndrome: A case report and review of literature
Abstract
Background: Myelodysplastic syndrome (MDS) is caused by malignant proliferation and ineffective hematopoiesis. Oncogenic somatic mutations and increased apoptosis, necroptosis and pyroptosis lead to the accumulation of earlier hematopoietic progenitors and impaired productivity of mature blood cells. An increased percentage of myeloblasts and the presence of unfavorable somatic mutations are signs of leukemic hematopoiesis and indicators of entrance into an advanced stage. Bone marrow cellularity and myeloblasts usually increase with disease progression. However, aplastic crisis occasionally occurs in advanced MDS.
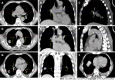
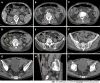
Case summary: A 72-year-old male patient was definitively diagnosed with MDS with excess blasts-1 (MDS-EB-1) based on an increase in the percentages of myeloblasts and cluster of differentiation (CD)34+ hematopoietic progenitors and the identification of myeloid neoplasm-associated somatic mutations in bone marrow samples. The patient was treated with hypomethylation therapy and was able to maintain a steady disease state for 2 years. In the treatment process, the advanced MDS patient experienced an episode of progressive pancytopenia and bone marrow aplasia. During the aplastic crisis, the bone marrow was infiltrated with sparsely distributed atypical lymphocytes. Surprisingly, the leukemic cells disappeared. Immunological analysis revealed that the atypical lymphocytes expressed a high frequency of CD3, CD5, CD8, CD16, CD56 and CD57, suggesting the activation of autoimmune cytotoxic T-lymphocytes and natural killer (NK)/NKT cells that suppressed both normal and leukemic hematopoiesis. Elevated serum levels of inflammatory cytokines, including interleukin (IL)-6, interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), confirmed the deranged type I immune responses. This morphological and immunological signature led to the diagnosis of severe aplastic anemia secondary to large granule lymphocyte leukemia. Disseminated tuberculosis was suspected upon radiological examinations in the search for an inflammatory niche. Antituberculosis treatment led to reversion of the aplastic crisis, disappearance of the atypical lymphocytes, increased marrow cellularity and 2 mo of hematological remission, providing strong evidence that disseminated tuberculosis was responsible for the development of the aplastic crisis, the regression of leukemic cells and the activation of CD56+ atypical lymphocytes. Reinstitution of hypomethylation therapy in the following 19 mo allowed the patient to maintain a steady disease state. However, the patient transformed the disease phenotype into acute myeloid leukemia and eventually died of disease progression and an overwhelming infectious episode.
Conclusion: Disseminated tuberculosis can induce CD56+ lymphocyte infiltration in the bone marrow and in turn suppress both normal and leukemic hematopoiesis, resulting in the development of aplastic crisis and leukemic cell regression.
Keywords: Aplastic crisis; Atypical lymphocyte; CD56+ lymphocyte expansion; Case report; Disseminated tuberculosis; Leukemic cell regression; Myelodysplastic syndrome.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflicts of interest to declare that are relevant to the content of this article.
Figures

Similar articles
-
When inflammatory stressors dramatically change, disease phenotypes may transform between autoimmune hematopoietic failure and myeloid neoplasms.Front Immunol. 2024 Feb 15;15:1339971. doi: 10.3389/fimmu.2024.1339971. eCollection 2024. Front Immunol. 2024. PMID: 38426096 Free PMC article. Review.
-
Leukemic transformation during anti-tuberculosis treatment in aplastic anemia-paroxysmal nocturnal hemoglobinuria syndrome: A case report and review of literature.World J Clin Cases. 2023 Oct 6;11(28):6908-6919. doi: 10.12998/wjcc.v11.i28.6908. World J Clin Cases. 2023. PMID: 37901004 Free PMC article.
-
Flared inflammatory episode transforms advanced myelodysplastic syndrome into aplastic pancytopenia: A case report and literature review.World J Clin Cases. 2023 Jun 16;11(17):4105-4116. doi: 10.12998/wjcc.v11.i17.4105. World J Clin Cases. 2023. PMID: 37388797 Free PMC article.
-
Special Education: Aplastic Anemia.Oncologist. 1996;1(3):187-189. Oncologist. 1996. PMID: 10387986
-
Immunologic aspects of hypoplastic myelodysplastic syndrome.Semin Oncol. 2011 Oct;38(5):667-72. doi: 10.1053/j.seminoncol.2011.04.006. Semin Oncol. 2011. PMID: 21943673 Free PMC article. Review.
Cited by
-
When inflammatory stressors dramatically change, disease phenotypes may transform between autoimmune hematopoietic failure and myeloid neoplasms.Front Immunol. 2024 Feb 15;15:1339971. doi: 10.3389/fimmu.2024.1339971. eCollection 2024. Front Immunol. 2024. PMID: 38426096 Free PMC article. Review.
-
Leukemic transformation during anti-tuberculosis treatment in aplastic anemia-paroxysmal nocturnal hemoglobinuria syndrome: A case report and review of literature.World J Clin Cases. 2023 Oct 6;11(28):6908-6919. doi: 10.12998/wjcc.v11.i28.6908. World J Clin Cases. 2023. PMID: 37901004 Free PMC article.
References
-
- Li H, Hu F, Gale RP, Sekeres MA, Liang Y. Myelodysplastic syndromes. Nat Rev Dis Primers. 2022;8:74. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

