Molecular signalling during cross talk between gut brain axis regulation and progression of irritable bowel syndrome: A comprehensive review
- PMID: 37469740
- PMCID: PMC10353503
- DOI: 10.12998/wjcc.v11.i19.4458
Molecular signalling during cross talk between gut brain axis regulation and progression of irritable bowel syndrome: A comprehensive review
Abstract
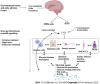
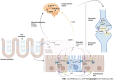
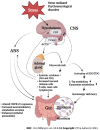
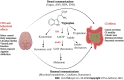
Irritable bowel syndrome (IBS) is a chronic functional disorder which alters gastrointestinal (GI) functions, thus leading to compromised health status. Pathophysiology of IBS is not fully understood, whereas abnormal gut brain axis (GBA) has been identified as a major etiological factor. Recent studies are suggestive for visceral hyper-sensitivity, altered gut motility and dysfunctional autonomous nervous system as the main clinical abnormalities in IBS patients. Bidirectional signalling interactions among these abnormalities are derived through various exogenous and endogenous factors, such as microbiota population and diversity, microbial metabolites, dietary uptake, and psychological abnormalities. Strategic efforts focused to study these interactions including probiotics, antibiotics and fecal transplantations in normal and germ-free animals are clearly suggestive for the pivotal role of gut microbiota in IBS etiology. Additionally, neurotransmitters act as communication tools between enteric microbiota and brain functions, where serotonin (5-hydroxytryptamine) plays a key role in pathophysiology of IBS. It regulates GI motility, pain sense and inflammatory responses particular to mucosal and brain activity. In the absence of a better understanding of various interconnected crosstalks in GBA, more scientific efforts are required in the search of novel and targeted therapies for the management of IBS. In this review, we have summarized the gut microbial composition, interconnected signalling pathways and their regulators, available therapeutics, and the gaps needed to fill for a better management of IBS.
Keywords: Gut brain axis; Irritable bowel syndrome; Microbiota; Serotonin; Stress.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures
References
-
- Leite G, Pimentel M, Barlow GM, Chang C, Hosseini A, Wang J, Parodi G, Sedighi R, Rezaie A, Mathur R. Age and the aging process significantly alter the small bowel microbiome. Cell Rep. 2021;36:109765. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

