CDKN1C gene mutation causing familial Silver-Russell syndrome: A case report and review of literature
- PMID: 37469742
- PMCID: PMC10353515
- DOI: 10.12998/wjcc.v11.i19.4655
CDKN1C gene mutation causing familial Silver-Russell syndrome: A case report and review of literature
Abstract
Background: Cyclin-dependent kinase inhibitor 1C (CDKN1C) is a cell proliferation inhibitor that regulates the cell cycle and cell growth through G1 cell cycle arrest. CDKN1C mutations can lead to IMAGe syndrome (CDKN1C allele gain-of-function mutations lead to intrauterine growth restriction, metaphyseal dysplasia, adrenal hypoplasia congenital, and genitourinary malformations). We present a Silver-Russell syndrome (SRS) pedigree that was due to a missense mutation affecting the same amino acid position, 279, in the CDKN1C gene, resulting in the amino acid substitution p.Arg279His (c.836G>A). The affected family members had an SRS phenotype but did not have limb asymmetry or adrenal insufficiency. The amino acid changes in this specific region were located in a narrow functional region that contained mutations previously associated with IMAGe syndrome. In familial SRS patients, the PCNA region of CDKN1C should be analysed. Adrenal insufficiency should be excluded in all patients with functional CDKN1C variants.
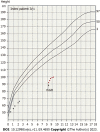
Case summary: We describe the case of an 8-year-old girl who initially presented with short stature. Her height was 91.6 cm, and her weight was 10.2 kg. Physical examination revealed that she had a relatively large head, an inverted triangular face, a protruding forehead, a low ear position, sunken eye sockets, and irregular cracked teeth but no limb asymmetry. Family history: The girl's mother, great-grandmother, and grandmother's brother also had a prominent forehead, triangular face, and severely proportional dwarfism but no limb asymmetry or adrenal insufficiency. Exome sequencing of the girl revealed a new heterozygous CDKN1C (NM_000076. 2) c.836G>A mutation, resulting in a variant with a predicted evolutionarily highly conserved arginine substituted by histidine (p.Arg279His). The same causative mutation was found in both the proband's mother, great-grandmother, and grandmother's brother, who had similar phenotypes. Thus far, we found an SRS pedigree, which was due to a missense mutation affecting the same amino acid position, 279, in the CDKN1C gene, resulting in the amino acid substitution p.Arg279His (c.836G>A). Although the SRS-related CDKN1C mutation is in the IMAGe-related mutation hotspot region [the proliferating cell nuclear antigen (PCNA) domain], no adrenal insufficiency was reported in this SRS pedigree. The reason may be that the location of the genomic mutation and the type of missense mutation determines the phenotype. The proband was treated with recombinant human growth hormone (rhGH). After 1 year of rhGH treatment, the height standard deviation score of the proband increased by 0.93 standard deviation score, and her growth rate was 8.1 cm/year. No adverse reactions, such as abnormal blood glucose, were found.
Conclusion: Functional mutations in CDKN1C can lead to familial SRS without limb asymmetry, and some patients may have glucose abnormalities. In familial SRS patients, the PCNA region of CDKN1C should be analysed. Adrenal insufficiency should be excluded in all patients with functional CDKN1C variants.
Keywords: CDKN1C; Case report; Gene; Mutation; Silver-Russell syndrome.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures



References
-
- Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. - PubMed
-
- Brioude F, Kalish JM, Mussa A, Foster AC, Bliek J, Ferrero GB, Boonen SE, Cole T, Baker R, Bertoletti M, Cocchi G, Coze C, De Pellegrin M, Hussain K, Ibrahim A, Kilby MD, Krajewska-Walasek M, Kratz CP, Ladusans EJ, Lapunzina P, Le Bouc Y, Maas SM, Macdonald F, Õunap K, Peruzzi L, Rossignol S, Russo S, Shipster C, Skórka A, Tatton-Brown K, Tenorio J, Tortora C, Grønskov K, Netchine I, Hennekam RC, Prawitt D, Tümer Z, Eggermann T, Mackay DJG, Riccio A, Maher ER. Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: an international consensus statement. Nat Rev Endocrinol. 2018;14:229–249. - PMC - PubMed
-
- DeBaun MR, King AA, White N. Hypoglycemia in Beckwith-Wiedemann syndrome. Semin Perinatol. 2000;24:164–171. - PubMed
-
- Elliott M, Bayly R, Cole T, Temple IK, Maher ER. Clinical features and natural history of Beckwith-Wiedemann syndrome: presentation of 74 new cases. Clin Genet. 1994;46:168–174. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

