Electrolytic Seawater Mineralization and the Mass Balances That Demonstrate Carbon Dioxide Removal
- PMID: 37469756
- PMCID: PMC10353002
- DOI: 10.1021/acsestengg.3c00004
Electrolytic Seawater Mineralization and the Mass Balances That Demonstrate Carbon Dioxide Removal
Abstract
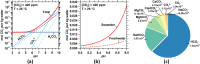
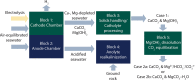
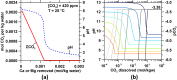
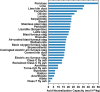
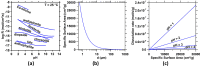
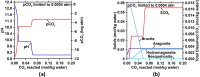
We present the mass balances associated with carbon dioxide (CO2) removal (CDR) using seawater as both the source of reactants and as the reaction medium via electrolysis following the "Equatic" (formerly known as "SeaChange") process. This process, extensively detailed in La Plante E.C.; ACS Sustain. Chem. Eng.2021, 9, ( (3), ), 1073-1089, involves the application of an electric overpotential that splits water to form H+ and OH- ions, producing acidity and alkalinity, i.e., in addition to gaseous coproducts, at the anode and cathode, respectively. The alkalinity that results, i.e., via the "continuous electrolytic pH pump" results in the instantaneous precipitation of calcium carbonate (CaCO3), hydrated magnesium carbonates (e.g., nesquehonite: MgCO3·3H2O, hydromagnesite: Mg5(CO3)4(OH)2·4H2O, etc.), and/or magnesium hydroxide (Mg(OH)2) depending on the CO32- ion-activity in solution. This results in the trapping and, hence, durable and permanent (at least ∼10 000-100 000 years) immobilization of CO2 that was originally dissolved in water, and that is additionally drawn down from the atmosphere within: (a) mineral carbonates, and/or (b) as solvated bicarbonate (HCO3-) and carbonate (CO32-) ions (i.e., due to the absorption of atmospheric CO2 into seawater having enhanced alkalinity). Taken together, these actions result in the net removal of ∼4.6 kg of CO2 per m3 of seawater catholyte processed. Geochemical simulations quantify the extents of net CO2 removal including the dependencies on the process configuration. It is furthermore indicated that the efficiency of realkalinization of the acidic anolyte using alkaline solids depends on their acid neutralization capacity and dissolution reactivity. We also assess changes in seawater chemistry resulting from Mg(OH)2 dissolution with emphasis on the change in seawater alkalinity and saturation state. Overall, this analysis provides direct quantifications of the ability of the Equatic process to serve as a means for technological CDR to mitigate the worst effects of accelerating climate change.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): E.C.L.P., X.C., D.J., L.C., D.S., and G.S. have an equity interest in Equatic Inc., which has licensed technology from UCLA that underpins this work.
Figures



References
-
- La Plante E. C.; Simonetti D. A.; Wang J.; Al-Turki A.; Chen X.; Jassby D.; Sant G. N. Saline Water-Based Mineralization Pathway for Gigatonne-Scale CO2 Management. ACS Sustain. Chem. Eng. 2021, 9 (3), 1073–1089. 10.1021/acssuschemeng.0c08561. - DOI
-
- Kelemen P. B.; McQueen N.; Wilcox J.; Renforth P.; Dipple G.; Vankeuren A. P. Engineered Carbon Mineralization in Ultramafic Rocks for CO2 Removal from Air: Review and New Insights. Chem. Geol. 2020, 550, 11962810.1016/j.chemgeo.2020.119628. - DOI
-
- Matter J. M.; Stute M.; Snæbjornsdottir S. O.; Oelkers E. H.; Gislason S. R.; Aradottir E. S.; Sigfusson B.; Gunnarsson I.; Sigurdardottir H.; Gunnlaugsson E.; Axelsson G.; Alfredsson H. A.; Wolff-Boenisch D.; Mesfin K.; Taya D. F. d. l. R.; Hall J.; Dideriksen K.; Broecker W. S. Rapid Carbon Mineralization for Permanent Disposal of Anthropogenic Carbon Dioxide Emissions. Science 2016, 352 (6291), 1312–1314. 10.1126/science.aad8132. - DOI - PubMed
-
- Mustafa J.; Mourad A. A.-H. I.; Al-Marzouqi A. H.; El-Naas M. H. Simultaneous Treatment of Reject Brine and Capture of Carbon Dioxide: A Comprehensive Review. Desalination 2020, 483, 11438610.1016/j.desal.2020.114386. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
