Endophytic fungus Colletotrichum sp. AP12 promotes growth physiology and andrographolide biosynthesis in Andrographis paniculata (Burm. f.) Nees
- PMID: 37469772
- PMCID: PMC10353853
- DOI: 10.3389/fpls.2023.1166803
Endophytic fungus Colletotrichum sp. AP12 promotes growth physiology and andrographolide biosynthesis in Andrographis paniculata (Burm. f.) Nees
Abstract
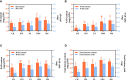
Endophytic fungi can promote host plant growth, enhance antioxidant defense enzyme activity, and induce the biosynthesis and accumulation of secondarymetabolites. Therefore, using endophytic fungi to improve the quality and yield of medicinal plants or important crops is an effective means of regulation. Colletotrichum sp. AP12 has been reported to produce andrographolide compounds (ADCs). This study aimed to investigate the effects of AP12 and its elicitors on the growth, defense enzyme activity, accumulation, and transcription levels of key genes in Andrographis paniculata (Burm. f.) Nees (A. paniculata). Using fermentation method to prepare AP12 into the inactivated fermentation solution (IFS), fermentation solution (FS), inactivated mycelium solution (IMS), and mycelium solution (MS), and the results showed that all four fungal elicitor components (ECs) could promote A. paniculata growth, enhance antioxidant defense enzymes, and increase ADC content and yield, especially the IMS group that had the highest leaf area, whole plant dry weight, superoxide dismutase (SOD), catalase (CAT) enzyme activities, total lactone contents, and yields, which were 2.37-, 1.60-, 2.20-, 3.27-, 1.59-, and 2.65-fold of the control, respectively. The 14-deoxyandrographolide (NAD) in the host irrigated with MS was 3.35-fold that of the control. In addition, AP12-infected A. paniculata sterile seedlings could significantly increase ADC content and expression levels of key enzyme genes, especially on day 12, when the total lactone content of the host reached 88.881± 5.793 mg/g DW, while on day 6, CPS gene expression level reached 10.79-fold that of the control, in turn promoting the biosynthesis and accumulation of andrographolide. In conclusion, the endophytic fungus AP12 is beneficial to the growth and secondary metabolism of A. paniculata, which is helpful for the cultivation and application of the biological bacterial fertilizer in A. paniculata, providing a theoretical and research basis for the use of endophytic fungi as a microbial resource to improve the quality and yield of medicinal plants.
Keywords: Andrographis paniculata (Burm. f.) Nees; Colletotrichum sp. AP12; andrographolide biosynthesis and accumulation; defense enzyme activity; elicitor components; plant growth.
Copyright © 2023 Xu, Li, Gu, Huang, Hu, Zheng, Hu and Du.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

