Optical Genome Mapping Identifies Novel Recurrent Structural Alterations in Childhood ETV6::RUNX1+ and High Hyperdiploid Acute Lymphoblastic Leukemia
- PMID: 37469802
- PMCID: PMC10353714
- DOI: 10.1097/HS9.0000000000000925
Optical Genome Mapping Identifies Novel Recurrent Structural Alterations in Childhood ETV6::RUNX1+ and High Hyperdiploid Acute Lymphoblastic Leukemia
Abstract
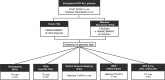
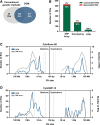
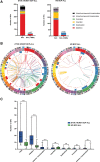
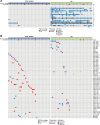
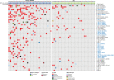
The mutational landscape of B-cell precursor acute lymphoblastic leukemia (BCP-ALL), the most common pediatric cancer, is not fully described partially because commonly applied short-read next generation sequencing has a limited ability to identify structural variations. By combining comprehensive analysis of structural variants (SVs), single-nucleotide variants (SNVs), and small insertions-deletions, new subtype-defining and therapeutic targets may be detected. We analyzed the landscape of somatic alterations in 60 pediatric patients diagnosed with the most common BCP-ALL subtypes, ETV6::RUNX1+ and classical hyperdiploid (HD), using conventional cytogenetics, single nucleotide polymorphism (SNP) array, whole exome sequencing (WES), and the novel optical genome mapping (OGM) technique. Ninety-five percent of SVs detected by cytogenetics and SNP-array were verified by OGM. OGM detected an additional 677 SVs not identified using the conventional methods, including (subclonal) IKZF1 deletions. Based on OGM, ETV6::RUNX1+ BCP-ALL harbored 2.7 times more SVs than HD BCP-ALL, mainly focal deletions. Besides SVs in known leukemia development genes (ETV6, PAX5, BTG1, CDKN2A), we identified 19 novel recurrently altered regions (in n ≥ 3) including 9p21.3 (FOCAD/HACD4), 8p11.21 (IKBKB), 1p34.3 (ZMYM1), 4q24 (MANBA), 8p23.1 (MSRA), and 10p14 (SFMBT2), as well as ETV6::RUNX1+ subtype-specific SVs (12p13.1 (GPRC5A), 12q24.21 (MED13L), 18q11.2 (MIB1), 20q11.22 (NCOA6)). We detected 3 novel fusion genes (SFMBT2::DGKD, PDS5B::STAG2, and TDRD5::LPCAT2), for which the sequence and expression were validated by long-read and whole transcriptome sequencing, respectively. OGM and WES identified double hits of SVs and SNVs (ETV6, BTG1, STAG2, MANBA, TBL1XR1, NSD2) in the same patient demonstrating the power of the combined approach to define the landscape of genomic alterations in BCP-ALL.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous
