Nitro-benzylideneoxymorphone, a bifunctional mu and delta opioid receptor ligand with high mu opioid receptor efficacy
- PMID: 37469877
- PMCID: PMC10352325
- DOI: 10.3389/fphar.2023.1230053
Nitro-benzylideneoxymorphone, a bifunctional mu and delta opioid receptor ligand with high mu opioid receptor efficacy
Abstract
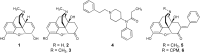
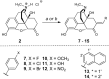
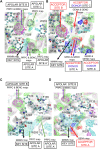
Introduction: There is a major societal need for analgesics with less tolerance, dependence, and abuse liability. Preclinical rodent studies suggest that bifunctional ligands with both mu (MOPr) and delta (DOPr) opioid peptide receptor activity may produce analgesia with reduced tolerance and other side effects. This study explores the structure-activity relationships (SAR) of our previously reported MOPr/DOPr lead, benzylideneoxymorphone (BOM) with C7-methylene-substituted analogs. Methods: Analogs were synthesized and tested in vitro for opioid receptor binding and efficacy. One compound, nitro-BOM (NBOM, 12) was evaluated for antinociceptive effects in the warm water tail withdrawal assay in C57BL/6 mice. Acute and chronic antinociception was determined, as was toxicologic effects on chronic administration. Molecular modeling experiments were performed using the Site Identification by Ligand Competitive Saturation (SILCS) method. Results: NBOM was found to be a potent MOPr agonist/DOPr partial agonist that produces high-efficacy antinociception. Antinociceptive tolerance was observed, as was weight loss; this toxicity was only observed with NBOM and not with BOM. Modeling supports the hypothesis that the increased MOPr efficacy of NBOM is due to the substituted benzylidene ring occupying a nonpolar region within the MOPr agonist state. Discussion: Though antinociceptive tolerance and non-specific toxicity was observed on repeated administration, NBOM provides an important new tool for understanding MOPr/DOPr pharmacology.
Keywords: SILCS; bifunctional analgesics; delta opioid receptor; dependence; mu opioid receptor; tolerance.
Copyright © 2023 Olson, Devereaux, Chatterjee, Saldaña-Shumaker, Shafer, Plotkin, Kandasamy, MacKerell, Traynor and Cunningham.
Conflict of interest statement
AM is cofounder and CSO of SilcsBio LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Aiming at Ideal Therapeutics-MOPr/DOPr or MOPr-DOPr Heteromertargeting Ligand.Curr Top Med Chem. 2020;20(31):2843-2851. doi: 10.2174/1568026620666200423095231. Curr Top Med Chem. 2020. PMID: 32324516 Review.
-
Development of a bioavailable μ opioid receptor (MOPr) agonist, δ opioid receptor (DOPr) antagonist peptide that evokes antinociception without development of acute tolerance.J Med Chem. 2014 Apr 10;57(7):3148-53. doi: 10.1021/jm5002088. Epub 2014 Mar 26. J Med Chem. 2014. PMID: 24641190 Free PMC article.
-
Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: implications for therapeutic applications.J Pharmacol Exp Ther. 2009 Dec;331(3):954-64. doi: 10.1124/jpet.109.157446. Epub 2009 Sep 22. J Pharmacol Exp Ther. 2009. PMID: 19773529 Free PMC article.
-
Behavioral effects of benzylideneoxymorphone (BOM), a low efficacy µ opioid receptor agonist and a δ opioid receptor antagonist.Psychopharmacology (Berl). 2020 Dec;237(12):3591-3602. doi: 10.1007/s00213-020-05638-1. Epub 2020 Aug 21. Psychopharmacology (Berl). 2020. PMID: 32820390
-
[MOPr-DOPr heteromer: the meaning and possibility as novel therapeutic target for pain control].Nihon Yakurigaku Zasshi. 2021;156(3):134-138. doi: 10.1254/fpj.20103. Nihon Yakurigaku Zasshi. 2021. PMID: 33952839 Review. Japanese.
Cited by
-
Exploring Druggable Binding Sites on the Class A GPCRs Using the Residue Interaction Network and Site Identification by Ligand Competitive Saturation.ACS Omega. 2024 Sep 13;9(38):40154-40171. doi: 10.1021/acsomega.4c06172. eCollection 2024 Sep 24. ACS Omega. 2024. PMID: 39346853 Free PMC article.
-
Enhancing SILCS-MC via GPU Acceleration and Ligand Conformational Optimization with Genetic and Parallel Tempering Algorithms.J Phys Chem B. 2024 Aug 1;128(30):7362-7375. doi: 10.1021/acs.jpcb.4c03045. Epub 2024 Jul 20. J Phys Chem B. 2024. PMID: 39031121 Free PMC article.
References
-
- Abdelhamid E. E., Sultana M., Portoghese P. S., Takemori A. E. (1991). Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J. Pharmacol. Exp. Ther. 258, 299–303. - PubMed
-
- Anand J. P., Kochan K. E., Nastase A. F., Montgomery D., Griggs N. W., Traynor J. R., et al. (2018). In vivo effects of μ-opioid receptor agonist/δ-opioid receptor antagonist peptidomimetics following acute and repeated administration. Br. J. Pharmacol. 175, 2013–2027. 10.1111/bph.14148 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

