The key role of pretreatment for the one-step and multi-step conversions of European lignocellulosic materials into furan compounds
- PMID: 37469965
- PMCID: PMC10352963
- DOI: 10.1039/d3ra01533e
The key role of pretreatment for the one-step and multi-step conversions of European lignocellulosic materials into furan compounds
Abstract
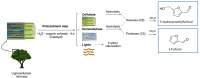
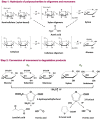
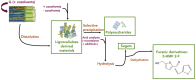
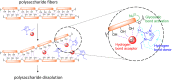
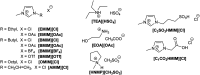
Nowadays, an increased interest from the chemical industry towards the furanic compounds production, renewable molecules alternatives to fossil molecules, which can be transformed into a wide range of chemicals and biopolymers. These molecules are produced following hexose and pentose dehydration. In this context, lignocellulosic biomass, owing to its richness in carbohydrates, notably cellulose and hemicellulose, can be the starting material for monosaccharide supply to be converted into bio-based products. Nevertheless, processing biomass is essential to overcome the recalcitrance of biomass, cellulose crystallinity, and lignin crosslinked structure. The previous reports describe only the furanic compound production from monosaccharides, without considering the starting raw material from which they would be extracted, and without paying attention to raw material pretreatment for the furan production pathway, nor the mass balance of the whole process. Taking account of these shortcomings, this review focuses, firstly, on the conversion potential of different European abundant lignocellulosic matrices into 5-hydroxymethyl furfural and 2-furfural based on their chemical composition. The second line of discussion is focused on the many technological approaches reported so far for the conversion of feedstocks into furan intermediates for polymer technology but highlighting those adopting the minimum possible steps and with the lowest possible environmental impact. The focus of this review is to providing an updated discussion of the important issues relevant to bringing chemically furan derivatives into a market context within a green European context.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

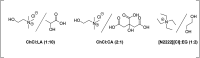
References
-
- Manzanares P. The role of biorefinering research in the development of a mpder n bioeconomy. Acta Innov. 2020;37:47–56.
-
- Gandini A. Lacerda T. M. Carvalho A. J. F. Trovatti E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016;116:1637–1669. - PubMed
-
- Van Putten R. J. et al., Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013;113:1499–1597. - PubMed
-
- Scapini T. et al., Hydrothermal pretreatment of lignocellulosic biomass for hemicellulose recovery. Bioresour. Technol. 2021;342:126033. - PubMed
-
- Ruiz H. A. et al., Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour. Technol. 2020;299:122685. - PubMed

