Ribonucleic Acid-Mediated Control of Protein Translation Under Stress
- PMID: 37470212
- PMCID: PMC10443204
- DOI: 10.1089/ars.2023.0233
Ribonucleic Acid-Mediated Control of Protein Translation Under Stress
Abstract
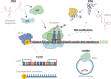
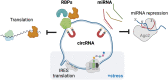
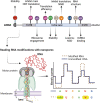
Significance: The need of cells to constantly respond to endogenous and exogenous stress has necessitated the evolution of pathways to counter the deleterious effects of stress and to restore cellular homeostasis. The inability to activate a timely and adequate response can lead to disease and is a hallmark of aging. Besides protein-coding genes, cells contain a plethora of noncoding regulatory elements that allow cells to respond rapidly and efficiently to external stimuli by activating highly specific and tightly controlled mechanisms. Many of these programs converge on the regulation of translation, one of the most energy-consuming processes in cells. Recent Advances: The noncoding dimension of translational regulation includes short and long noncoding ribonucleic acids (ncRNAs), as well as messenger RNA features, such as the sequence and modification status of the 5' and 3' untranslated regions (UTRs), that do not change the amino acid sequence of the produced protein. Critical Issues: In this review, we discuss the regulatory role of the nonprotein-coding components of translation under stress, particularly oxidative stress. We conclude that the regulation of translation through ncRNAs, UTRs, and nucleotide modifications is emerging as a critical component of the stress response. Future Directions: Further areas of study using long-read sequencing technologies will be discussed. Antioxid. Redox Signal. 39, 374-389.
Keywords: RNA modifications; noncoding RNA; stress; translation.
Conflict of interest statement
No competing financial interests exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

