Patient Characteristics, Microbiology, and Mortality of Infective Endocarditis After Transcatheter Aortic Valve Implantation
- PMID: 37470442
- PMCID: PMC10724461
- DOI: 10.1093/cid/ciad431
Patient Characteristics, Microbiology, and Mortality of Infective Endocarditis After Transcatheter Aortic Valve Implantation
Abstract
Background: Infective endocarditis (IE) after transcatheter aortic valve implantation (TAVI) is associated with high mortality and surgery is rarely performed. Thus, to inform on preventive measures and treatment strategies, we investigated patient characteristics and microbiology of IE after TAVI.
Methods: Using Danish nationwide registries, we identified patients with IE after TAVI, IE after non-TAVI prosthetic valve (nTPV), and native valve IE. Patient characteristics; overall, early (≤12 m), and late IE (>12 m) microbiology; and unadjusted and adjusted mortality were compared.
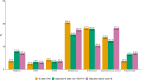
Results: We identified 273, 1022, and 5376 cases of IE after TAVI, IE after nTPV, and native valve IE. Age and frailty were highest among TAVI IE (4.8%; median age: 82 y; 61.9% frail). Enterococcus spp. were common for IE after TAVI (27.1%) and IE after nTPV (21.2%) compared with native valve IE (11.4%). Blood culture-negative IE was rare in IE after TAVI (5.5%) compared with IE after nTPV (15.2%) and native valve IE (13.5%). The unadjusted 90-day mortality was comparable, but the 5-year mortality was highest for IE after TAVI (75.2% vs 57.2% vs 53.6%). In Cox models adjusted for patient characteristics and bacterial etiology for 1-90 days and 91-365 days, there was no significant difference in mortality rates.
Conclusions: Patients with IE after TAVI are older and frailer, enterococci and streptococci are often the etiologic agents, and are rarely blood culture negative compared with other IE patients. Future studies regarding antibiotic prophylaxis strategies covering enterococci should be considered in this setting.
Keywords: epidemiology; infective endocarditis; microbiology; outcomes; transcatheter aortic valve implantation.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. L. K.: Lecture fees from AstraZeneca, Bayer, Boehringer, Novartis, and Novo. H. B.: Lecture fees from Amgen, MSD, Bristol-Myers Squibb, and Sanofi. M. V.: Lecture fees from MyLab Oy. J. B. O.: Speaker’s honoraria or consultancy fees from Bayer, Bristol-Myers Squibb, and Pfizer. E. L. F.: Independent research grant from Novo Nordisk Foundation. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

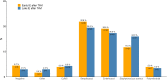


References
-
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2021; 143:e227. - PubMed
-
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022; 43:561–632. - PubMed
-
- Østergaard L, Lauridsen TK, Iversen K, et al. Infective endocarditis in patients who have undergone transcatheter aortic valve implantation: a review. Clin Microbiol Infect 2020; 26:999–1007. - PubMed
-
- del Val D, Panagides V, Mestres CA, Miró JM, Rodés-Cabau J. Infective endocarditis after transcatheter aortic valve replacement. J Am Coll Cardiol 2023; 81:394–412. - PubMed
-
- Durko AP, Osnabrugge RL, van Mieghem NM, et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J 2018; 39:2635–42. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

