Anti-PLA2R Antibody Levels and Clinical Risk Factors for Treatment Nonresponse in Membranous Nephropathy
- PMID: 37471101
- PMCID: PMC10578640
- DOI: 10.2215/CJN.0000000000000237
Anti-PLA2R Antibody Levels and Clinical Risk Factors for Treatment Nonresponse in Membranous Nephropathy
Abstract
Background: The 2021 Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend following anti-phospholipase A2 receptor (PLA2R) antibody levels as a marker of treatment response in membranous nephropathy; however, the optimal timing to evaluate antibody levels and how to combine them with other clinical variables are currently unknown.
Methods: We used a cohort of 85 patients from the Membranous Nephropathy Trial Of Rituximab (MENTOR) with anti-PLA2R antibodies ≥14 RU/ml to identify risk factors for not experiencing proteinuria remission after 12 months of treatment with cyclosporine or rituximab. Three landmark times were considered: at baseline and after 3 and 6 months of treatment. Logistic regression model performance was evaluated using C-statistics and model fit (Akaike information criterion [AIC], R 2 ).
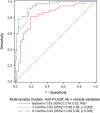
Results: The model at baseline that best predicted no remission included anti-PLA2R antibodies >323 RU/ml and creatinine clearance; the best model after 3 months included the change from baseline in both antibody and albumin levels; and the best model after 6 months included antibody levels >14 RU/ml, creatinine clearance, and the change from baseline in albumin. Compared with the model at baseline, the model at 3 months had better model fit (AIC 70.9 versus 96.4, R 2 51.8% versus 30.1%) and higher C-statistic (0.93 versus 0.83, P = 0.008). The model at 6 months had no difference in performance compared with the model at 3 months (AIC 68.6, R 2 53.0%, C-statistic 0.94, P = 0.67).
Conclusions: In patients with membranous nephropathy treated with cyclosporine or rituximab in the MENTOR trial, we found that the optimal method to evaluate risk factors for the probability of treatment response was to use anti-PLA2R antibody levels combined with albumin levels after 3 months of treatment, which was significantly better than using antibody levels alone or risk factor evaluation at baseline, with no added benefit of waiting until 6 months of treatment.
Podcast: This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/CJASN/2023_10_09_CJN0000000000000237.mp3.
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
G.B. Appel reports consultancy for Achillion, Alexion, Apellis, Arrowhead, Aurinia, Bristol Myers Squibb, Chemocentryx, Chinook, EMD Serono, Genentech, Genzyme-Sanofi, GlaxoSmithKline, E. Lilly, Mallinkrodt, Merck, Novartis, Omeros, Pfizer, Reata, Travere Therapeutics, and Vertex Therapeutics; research funding from Achillion-Alexion, Apellis, Calliditas, Chemocentryx, Equillium, Genentech-Roche, Goldfinch, Mallinkrodt, Novartis, Reata, Sanofi-Genzyme, and Vertex—all through Columbia University; honoraria from Aurinia, Calliditas, and GlaxoSmithKline; royalties from UpToDate; advisory or leadership roles for UpToDate Editorial Board and Med Advisory board for Alexion, Alexion-Achillion, Apellis, Arrowhead, Aurinia, BM Squib, Chinook, Genentech, GlaxoSmithKline, Lilly, Reata, Roche, and Sanofi—no role as officer or board member of any pharmaceutical or other; and speakers bureau for Aurinia lectures on lupus nephritis, for GSK for lectures on lupus nephritis, and Calliditas for lecture on the gut and IgAN. N. Aslam reports ownership interest in Doximity; research funding from AstraZeneca, Baxter, Idorsia, Novartis, and Otsuka; and advisory or leadership roles for Chinook Advisory Board, Florida Society of Nephrology Board of Directors, and Travere Therapeutics Advisory Board. S.J. Barbour reports consultancy for Achillion, Alexion, Eledon, HIBio, Inception Sciences, Novartis, Pfizer, Vera, and Visterra; research funding from Alexion, Novartis, and Roche; and honoraria from Alexion and Roche. D.C. Cattran reports consultancy for Alexi, Alnylam, Aurinia, Calliditis, Chemocentrx, Chinook Therapeutics, Forsee, Horizon, Reistone, Vera Therapeutics, and Zyversa Therapeutics; research funding from Alnylam; honoraria from Alexion, Calliditis, and Kyowa Hakko Kirin Co; advisory or leadership roles for Alnylam, Calliditis, NephCure, SONG-GD, UpToDate, and Vera; and other interests or relationships with Aurinea, Dimerix, Novartis, and Vera Therapeutics. F.C. Fervenza reports employment with Mayo Clinic; consultancy for Alexion Pharmaceuticals, ByoCrystal, Galapagos, GSK, Novartis, Otsuka, and Takeda; research funding from Chemocentryx, Genentech, Hoffman La Roche, Janssen Pharmaceutical, Morphosys, and Retrophin; honoraria from UpToDate; and advisory or leadership roles for
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

