A Mobile Clinical Decision Support System for High-Risk Pregnant Women in Rural India (SMARThealth Pregnancy): Pilot Cluster Randomized Controlled Trial
- PMID: 37471135
- PMCID: PMC10401191
- DOI: 10.2196/44362
A Mobile Clinical Decision Support System for High-Risk Pregnant Women in Rural India (SMARThealth Pregnancy): Pilot Cluster Randomized Controlled Trial
Abstract
Background: Cardiovascular disease (CVD) is the leading cause of death in women in India. Early identification is crucial to reducing deaths. Hypertensive disorders of pregnancy (HDP) and gestational diabetes mellitus (GDM) carry independent risks for future CVD, and antenatal care is a window to screen and counsel high-risk women. In rural India, community health workers (CHWs) deliver antenatal and postnatal care. We developed a complex intervention (SMARThealth Pregnancy) involving mobile clinical decision support for CHWs and evaluated it in a pilot cluster randomized controlled trial (cRCT).
Objective: The aim of the study is to co-design a theory-informed intervention for CHWs to screen, refer, and counsel pregnant women at high risk of future CVD in rural India and evaluate its feasibility and acceptability.
Methods: In phase 1, we used qualitative methods to explore community priorities for high-risk pregnant women in rural areas of 2 diverse states in India. In phase 2, informed by behavior change theory and human-centered design, we used these qualitative data to develop the intervention components and implementation strategies for SMARThealth Pregnancy in an iterative process with end users. In phase 3, using mixed methods, we evaluated the intervention in a cRCT with an embedded qualitative substudy across 4 primary health centres: 2 in Jhajjar district, Haryana, and 2 in Guntur district, Andhra Pradesh.
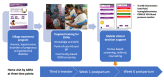
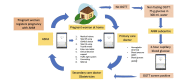
Results: SMARThealth Pregnancy embedded a total of 15 behavior change techniques and included (1) community awareness programs; (2) targeted training, including point-of-care blood pressure and hemoglobin measurement; and (3) mobile clinical decision support for CHWs to screen women in their homes. The intervention focused on 3 priority conditions: anemia, HDP, and GDM. The evaluation involved a total of 200 pregnant women, equally randomized to intervention or enhanced standard care (control). Recruitment was completed within 5 months, with minimal loss to follow-up (4/200, 2%) at 6 weeks postpartum. A total of 4 primary care doctors and 54 CHWs in the intervention clusters took part in the study. Fidelity to intervention practices was 100% prepandemic. Over half the study population was affected by moderate to severe anemia at baseline. The prevalence of HDP (2.5%) and GDM (2%) was low in our study population. Results suggest a possible improvement in mean hemoglobin (anemia) in the intervention group, although an adequately powered trial is needed. The model of home-based care was feasible and acceptable for pregnant or postpartum women and CHWs, who perceived improvements in quality of care, self-efficacy, and professional recognition.
Conclusions: SMARThealth Pregnancy is an innovative model of home-based care for high-risk pregnant women during the transitions between antenatal and postnatal care and adult health services. The use of theory and co-design during intervention development facilitated acceptability of the intervention and implementation strategies. Our experience has informed the decision to initiate a larger-scale cRCT.
Trial registration: ClinicalTrials.gov NCT03968952; https://clinicaltrials.gov/ct2/show/NCT03968952.
International registered report identifier (irrid): RR2-10.3389/fgwh.2021.620759.
Keywords: cardiovascular diseases; clinical; community health workers; decision support systems; diabetes; gestational; high risk; pregnancy; telemedicine.
©Shobhana Nagraj, Stephen Kennedy, Vivekananda Jha, Robyn Norton, Lisa Hinton, Laurent Billot, Eldho Rajan, Ameer Mohammed Abdul, Anita Phalswal, Varun Arora, Devarsetty Praveen, Jane Hirst. Originally published in JMIR Formative Research (https://formative.jmir.org), 20.07.2023.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures



References
-
- Prabhakaran D, Jeemon P, Sharma M, Roth GA, Johnson C, Harikrishnan S, Gupta R, Pandian JD, Naik N, Roy A, Dhaliwal RS, Xavier D, Kumar RK, Tandon N, Mathur P, Shukla DK, Mehrotra R, Venugopal K, Kumar GA, Varghese CM, Furtado M, Muraleedharan P, Abdulkader RS, Alam T, Anjana RM, Arora M, Bhansali A, Bhardwaj D, Bhatia E, Chakma JK, Chaturvedi P, Dutta E, Glenn S, Gupta PC, Johnson SC, Kaur T, Kinra S, Krishnan A, Kutz M, Mathur MR, Mohan V, Mukhopadhyay S, Nguyen M, Odell CM, Oommen AM, Pati S, Pletcher M, Prasad K, Rao PV, Shekhar C, Sinha DN, Sylaja PN, Thakur JS, Thankappan KR, Thomas N, Yadgir S, Yajnik CS, Zachariah G, Zipkin B, Lim SS, Naghavi M, Dandona R, Vos T, Murray CJL, Reddy KS, Swaminathan S, Dandona L. The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990–2016. Lancet Global Health. 2018 Dec;6(12):e1339–e1351. doi: 10.1016/s2214-109x(18)30407-8. - DOI - PMC - PubMed
-
- Norton R, Peters S, Jha V, Kennedy S, Woodward M. Women's health: a new global agenda. Oxford Martin School. 2016. [2023-06-27]. https://www.oxfordmartin.ox.ac.uk/downloads/briefings/women%27s-health.pdf . - PMC - PubMed
-
- Bellamy L, Casas J, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007 Nov 01;335(7627):974. doi: 10.1136/bmj.39335.385301.be. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

