Cell-free, high-density lipoprotein-specific phospholipid efflux assay predicts incident cardiovascular disease
- PMID: 37471145
- PMCID: PMC10503808
- DOI: 10.1172/JCI165370
Cell-free, high-density lipoprotein-specific phospholipid efflux assay predicts incident cardiovascular disease
Abstract
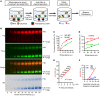
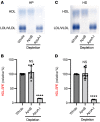
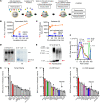
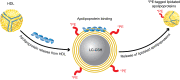
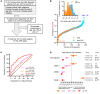
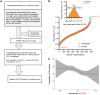
BACKGROUNDCellular cholesterol efflux capacity (CEC) is a better predictor of cardiovascular disease (CVD) events than HDL-cholesterol (HDL-C) but is not suitable as a routine clinical assay.METHODSWe developed an HDL-specific phospholipid efflux (HDL-SPE) assay to assess HDL functionality based on whole plasma HDL apolipoprotein-mediated solubilization of fluorescent phosphatidylethanolamine from artificial lipid donor particles. We first assessed the association of HDL-SPE with prevalent coronary artery disease (CAD): study I included NIH severe-CAD (n = 50) and non-CAD (n = 50) participants, who were frequency matched for sex, BMI, type 2 diabetes mellitus, and smoking; study II included Japanese CAD (n = 70) and non-CAD (n = 154) participants. We also examined the association of HDL-SPE with incident CVD events in the Prevention of Renal and Vascular End-stage Disease (PREVEND) study comparing 340 patients with 340 controls individually matched for age, sex, smoking, and HDL-C levels.RESULTSReceiver operating characteristic curves revealed stronger associations of HDL-SPE with prevalent CAD. The AUCs in study I were as follows: HDL-SPE, 0.68; apolipoprotein A-I (apoA-I), 0.62; HDL-C, 0.63; and CEC, 0.52. The AUCs in study II were as follows: HDL-SPE, 0.83; apoA-I, 0.64; and HDL-C, 0.53. Also longitudinally, HDL-SPE was significantly associated with incident CVD events independent of traditional risk factors with ORs below 0.2 per SD increment in the PREVEND study (P < 0.001).CONCLUSIONHDL-SPE could serve as a routine clinical assay for improving CVD risk assessment and drug discovery.TRIAL REGISTRATIONClinicalTrials.gov NCT01621594.FUNDINGNHLBI Intramural Research Program, NIH (HL006095-06).
Keywords: Cardiovascular disease; Lipoproteins; Molecular diagnosis; Vascular Biology.
Figures

References
-
- Grundy SM, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;73(24):e285–e1143. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

