IL-6 mediates the hepatic acute phase response after prerenal azotemia in a clinically defined murine model
- PMID: 37471421
- PMCID: PMC10511171
- DOI: 10.1152/ajprenal.00267.2022
IL-6 mediates the hepatic acute phase response after prerenal azotemia in a clinically defined murine model
Abstract
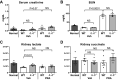
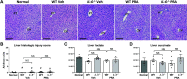
Prerenal azotemia (PRA) is a major cause of acute kidney injury and uncommonly studied in preclinical models. We sought to develop and characterize a novel model of PRA that meets the clinical definition: acute loss of glomerular filtration rate (GFR) that returns to baseline with resuscitation. Adult male C57BL/6J wild-type (WT) and IL-6-/- mice were studied. Intraperitoneal furosemide (4 mg) or vehicle was administered at time = 0 and 3 h to induce PRA from volume loss. Resuscitation began at 6 h with 1 mL intraperitoneal saline for four times for 36 h. Six hours after furosemide administration, measured glomerular filtration rate was 25% of baseline and returned to baseline after saline resuscitation at 48 h. After 6 h of PRA, plasma interleukin (IL)-6 was significantly increased, kidney and liver histology were normal, kidney and liver lactate were normal, and kidney injury molecule-1 immunofluorescence was negative. There were 327 differentially regulated genes upregulated in the liver, and the acute phase response was the most significantly upregulated pathway; 84 of the upregulated genes (25%) were suppressed in IL-6-/- mice, and the acute phase response was the most significantly suppressed pathway. Significantly upregulated genes and their proteins were also investigated and included serum amyloid A2, serum amyloid A1, lipocalin 2, chemokine (C-X-C motif) ligand 1, and haptoglobin; hepatic gene expression and plasma protein levels were all increased in wild-type PRA and were all reduced in IL-6-/- PRA. This work demonstrates previously unknown systemic effects of PRA that includes IL-6-mediated upregulation of the hepatic acute phase response.NEW & NOTEWORTHY Prerenal azotemia (PRA) accounts for a third of acute kidney injury (AKI) cases yet is rarely studied in preclinical models. We developed a clinically defined murine model of prerenal azotemia characterized by a 75% decrease in measured glomerular filtration rate (GFR), return of measured glomerular filtration rate to baseline with resuscitation, and absent tubular injury. Numerous systemic effects were observed, such as increased plasma interleukin-6 (IL-6) and upregulation of the hepatic acute phase response.
Keywords: NGAL; acute kidney injury; biomarkers; interleukin-6; organ cross talk; volume depletion.
Conflict of interest statement
S.F. receives consulting fees from SeaStar Medical. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures





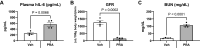






Similar articles
-
Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury.Hepatology. 2014 Aug;60(2):622-32. doi: 10.1002/hep.26980. Epub 2014 Jun 26. Hepatology. 2014. PMID: 24375576 Free PMC article.
-
IL-6-mediated hepatocyte production is the primary source of plasma and urine neutrophil gelatinase-associated lipocalin during acute kidney injury.Kidney Int. 2020 May;97(5):966-979. doi: 10.1016/j.kint.2019.11.013. Epub 2019 Nov 28. Kidney Int. 2020. PMID: 32081304 Free PMC article.
-
Mild elevation of urinary biomarkers in prerenal acute kidney injury.Kidney Int. 2012 Nov;82(10):1114-20. doi: 10.1038/ki.2012.266. Epub 2012 Aug 1. Kidney Int. 2012. PMID: 22854644
-
Clinical Application of Kidney Biomarkers in Cirrhosis.Am J Kidney Dis. 2020 Nov;76(5):710-719. doi: 10.1053/j.ajkd.2020.03.016. Epub 2020 Jul 1. Am J Kidney Dis. 2020. PMID: 32622560 Review.
-
Acute kidney injury in patients with cirrhosis: perils and promise.Clin Gastroenterol Hepatol. 2013 Dec;11(12):1550-8. doi: 10.1016/j.cgh.2013.03.018. Epub 2013 Apr 10. Clin Gastroenterol Hepatol. 2013. PMID: 23583467 Free PMC article. Review.
Cited by
-
Guideline-directed medical strategies for the co-management of heart failure and metabolic dysfunction-associated steatotic liver disease.Commun Med (Lond). 2025 Jul 28;5(1):312. doi: 10.1038/s43856-025-00951-2. Commun Med (Lond). 2025. PMID: 40721859 Free PMC article. Review.
-
Loss of SVIP Results in Metabolic Reprograming and Increased Retention of Very-Low-Density Lipoproteins in Hepatocytes.Int J Mol Sci. 2025 Aug 1;26(15):7465. doi: 10.3390/ijms26157465. Int J Mol Sci. 2025. PMID: 40806595 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

