C-2 Thiophenyl Tryptophan Trimers Inhibit Cellular Entry of SARS-CoV-2 through Interaction with the Viral Spike (S) Protein
- PMID: 37471688
- PMCID: PMC10424185
- DOI: 10.1021/acs.jmedchem.3c00576
C-2 Thiophenyl Tryptophan Trimers Inhibit Cellular Entry of SARS-CoV-2 through Interaction with the Viral Spike (S) Protein
Abstract
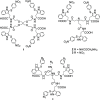
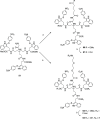
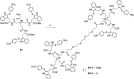
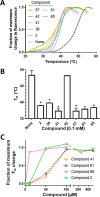
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes COVID-19, by infecting cells via the interaction of its spike protein (S) with the primary cell receptor angiotensin-converting enzyme (ACE2). To search for inhibitors of this key step in viral infection, we screened an in-house library of multivalent tryptophan derivatives. Using VSV-S pseudoparticles, we identified compound 2 as a potent entry inhibitor lacking cellular toxicity. Chemical optimization of 2 rendered compounds 63 and 65, which also potently inhibited genuine SARS-CoV-2 cell entry. Thermofluor and microscale thermophoresis studies revealed their binding to S and to its isolated receptor binding domain (RBD), interfering with the interaction with ACE2. High-resolution cryoelectron microscopy structure of S, free or bound to 2, shed light on cell entry inhibition mechanisms by these compounds. Overall, this work identifies and characterizes a new class of SARS-CoV-2 entry inhibitors with clear potential for preventing and/or fighting COVID-19.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Siegel D.; Hui H. C.; Doerffler E.; Clarke M. O.; Chun K.; Zhang L.; Neville S.; Carra E.; Lew W.; Ross B.; Wang Q.; Wolfe L.; Jordan R.; Soloveva V.; Knox J.; Perry J.; Perron M.; Stray K. M.; Barauskas O.; Feng J. Y.; Xu Y.; Lee G.; Rheingold A. L.; Ray A. S.; Bannister R.; Strickley R.; Swaminathan S.; Lee W. A.; Bavari S.; Cihlar T.; Lo M. K.; Warren T. K.; Mackman R. L. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][Triazin-4-Amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60, 1648–1661. 10.1021/acs.jmedchem.6b01594. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Medical
Miscellaneous

