Cell Surface Labeling and Detection of Protein Tyrosine Kinase 7 via Covalent Aptamers
- PMID: 37473438
- PMCID: PMC10401710
- DOI: 10.1021/jacs.3c02752
Cell Surface Labeling and Detection of Protein Tyrosine Kinase 7 via Covalent Aptamers
Abstract
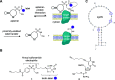
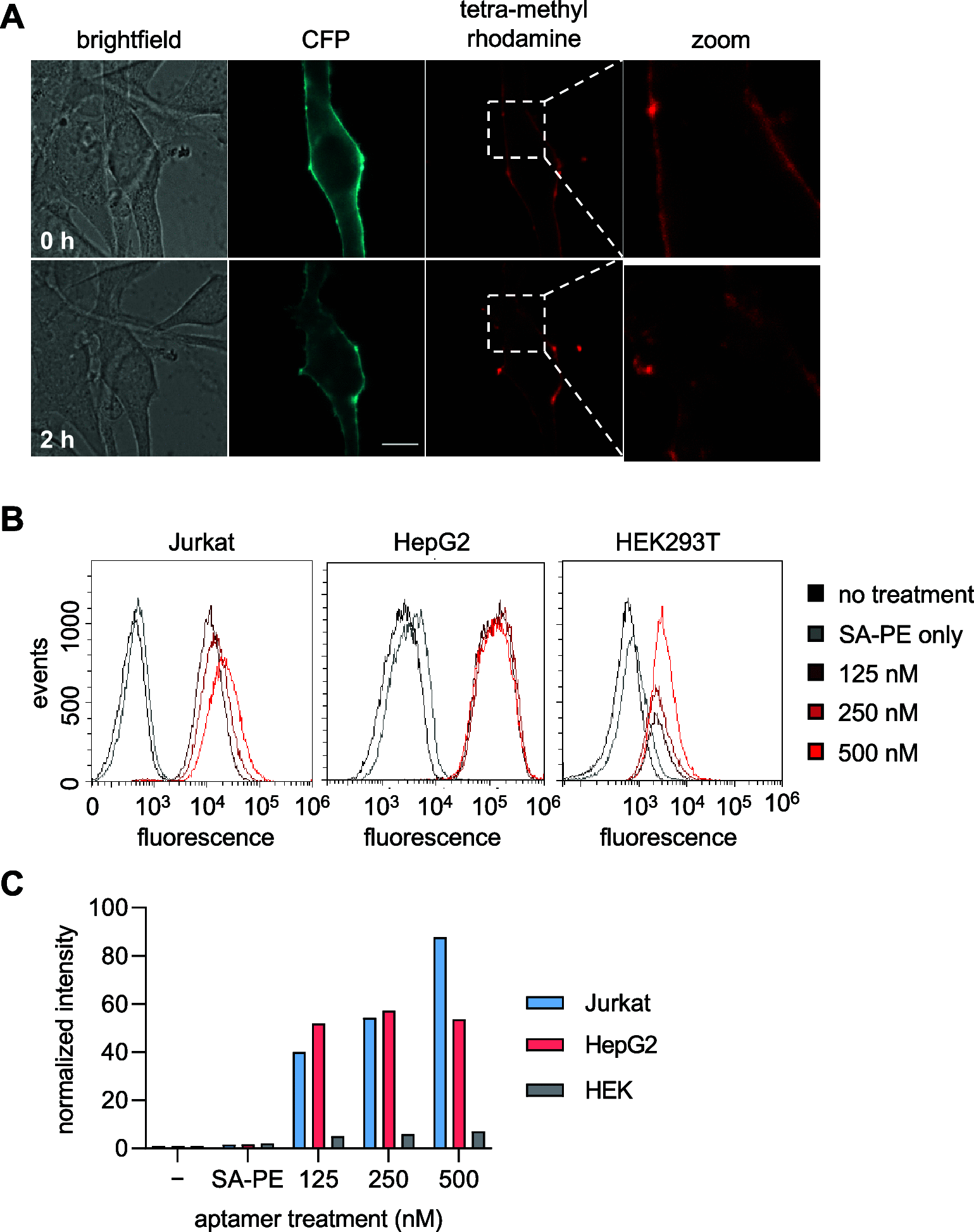
Covalent aptamers are novel biochemical tools for fast and selective transfer of labels to target proteins. Equipped with cleavable electrophiles, these nucleic acid probes enable the installation of functional handles onto native proteins. The high affinity and specificity with which aptamers bind their selected targets allows for quick, covalent labeling that can compete with nuclease-mediated degradation. Here, we introduce the first application of covalent aptamers to modify a specific cell surface protein through proximity-driven label transfer. We targeted protein tyrosine kinase 7 (PTK7), a prominent cancer marker, and demonstrated aptamer-mediated biotin transfer to specific lysine residues on the extracellular domain of the protein. This allowed for tracking of PTK7 expression, localization, and cellular internalization. These studies validate the programmability of covalent aptamers and highlight their applicability in a cellular context, including protein and small molecule delivery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Lhoumeau A.-C.; Martinez S.; Prébet T.; Borg J.-P.. The PTK7 Receptor Family. In Receptor Tyrosine Kinases: Family and Subfamilies; Wheeler D. L.; Yarden Y., Eds.; Springer International Publishing, 2015; pp. 539–558, 10.1007/978-3-319-11888-8_11. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

