Efficacy and Safety of Sucroferric Oxyhydroxide Compared with Sevelamer Carbonate in Chinese Dialysis Patients with Hyperphosphataemia: A Randomised, Open-Label, Multicentre, 12-Week Phase III Study
- PMID: 37473746
- PMCID: PMC10794965
- DOI: 10.1159/000531869
Efficacy and Safety of Sucroferric Oxyhydroxide Compared with Sevelamer Carbonate in Chinese Dialysis Patients with Hyperphosphataemia: A Randomised, Open-Label, Multicentre, 12-Week Phase III Study
Abstract
Introduction: This study aimed to investigate the efficacy and safety of sucroferric oxyhydroxide (SFOH) versus sevelamer carbonate in controlling serum phosphorus (sP) in adult Chinese dialysis patients with hyperphosphataemia (sP >1.78 mmol/L).
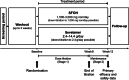
Methods: Open-label, randomised (1:1), active-controlled, parallel group, multicentre, phase III study of SFOH and sevelamer at starting doses corresponding to 1,500 mg iron/day and 2.4 g/day, respectively, with 8-week dose titration and 4-week maintenance (NCT03644264). Primary endpoint was non-inferiority analysis of change in sP from baseline to week 12. Secondary endpoints included sP over time and safety.
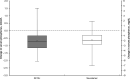
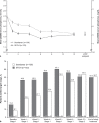
Results: 415 patients were screened; 286 were enrolled and randomised (142 and 144 to SFOH and sevelamer, respectively). Mean (SD) baseline sP: 2.38 (0.57) and 2.38 (0.52) mmol/L, respectively. Mean (SD) change in sP from baseline to week 12: - 0.71 (0.60) versus -0.63 (0.52) mmol/L, respectively; difference (sevelamer minus SFOH) in least squares means (95% CI): 0.08 mmol/L (-0.02, 0.18) with the lower limit of 95% CI above the non-inferiority margin of -0.34 mmol/L. The SFOH group achieved target sP (1.13-1.78 mmol/L) earlier than the sevelamer group (56.5% vs. 32.8% at week 4) and with a lower pill burden (mean 3.7 vs. 9.1 tablets/day over 4 weeks of maintenance, respectively). Safety and tolerability of SFOH was consistent with previous studies, and no new safety signals were observed.
Conclusion: SFOH effectively reduced sP from baseline and was non-inferior to sevelamer after 12 weeks of treatment but had a lower pill burden in Chinese dialysis patients with hyperphosphataemia; SFOH benefit-risk profile is favourable in Chinese patients.
Keywords: Chronic kidney disease; Hyperphosphataemia; Phosphate binder; Sucroferric oxyhydroxide.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
J.L. and L.Z. have no conflicts of interest to declare. S.W., L.B., M.M., and M.E. are employees of Vifor Pharma Management Ltd.
Figures

References
-
- Kidney Disease Improving Global Outcomes KDIGO CKD-MBD Update Work Group . KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017 Jul;7(1):1–59. 10.1016/j.kisu.2017.04.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

